Workshop on Polio updates End game strategies Organized




























- Slides: 58

Workshop on “Polio updates & End game strategies” Organized by Community Medicine Department, GMERS Medical College, Sola, in collaboration with NPSP (WHO), Gandhinagar 16 th April, 2013 National Polio Surveillance Project: Go. I & WHO

Polio update, AFP Surveillance End game strategy Dr. Anish Sinha State Surveillance Medical Officer World Health Organization National Polio Surveillance Project, India, Gandhinagar. National Polio Surveillance Project: Go. I & WHO

Contents…. • • • Global/ National / State update. Epidemiology of polio. AFP Surveillance. SIAs (NIDs / SNIDs). Certification of Polio eradication. End game strategy. National Polio Surveillance Project: Go. I & WHO

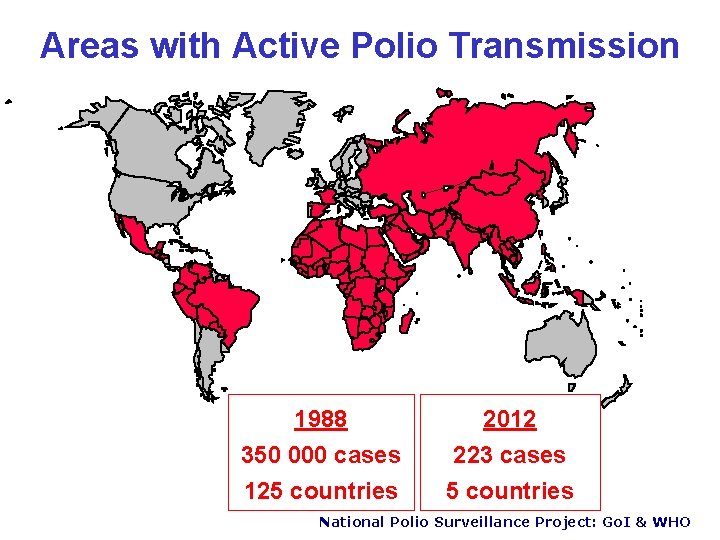
Areas with Active Polio Transmission 1988 350 000 cases 125 countries 2012 223 cases 5 countries National Polio Surveillance Project: Go. I & WHO

Rukhsar Khatoon last case of WPV detected in India (Jan 2011), her mother Shabida Bibi in Shahapar village, WB National Polio Surveillance Project: Go. I & WHO

India • India last polio case on 13 th Jan. 2011 • Removed from list of ENDEMIC Countries list in Feb. 2012 Looking forward for Certification (SEAR) in Feb. 2014 National Polio Surveillance Project: Go. I & WHO

Global Polio updates 2012 Nigeria (122) Pakistan (58) ONLY THREE ENDEMIC COUNTRIES Afghanistan (37) CHAD 5 WPV 1 (IMPORTATION) Niger 1 WPV 1 Till 9 Apr 13 – 18 cases (Nig-11, Pak-6, Afg-1) National Polio Surveillance Project: Go. I & WHO

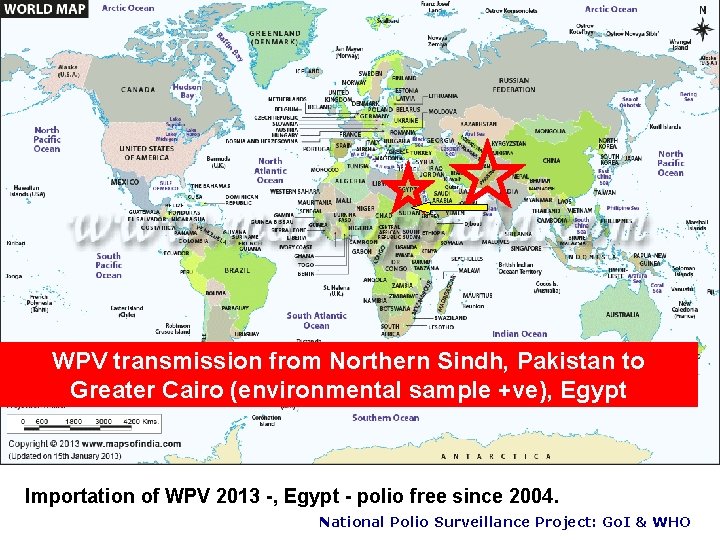
WPV transmission from Northern Sindh, Pakistan to Greater Cairo (environmental sample +ve), Egypt Importation of WPV 2013 -, Egypt - polio free since 2004. National Polio Surveillance Project: Go. I & WHO

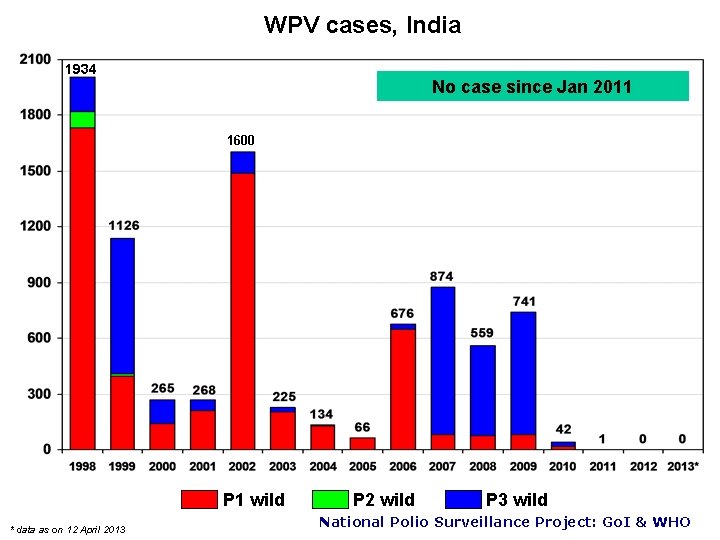
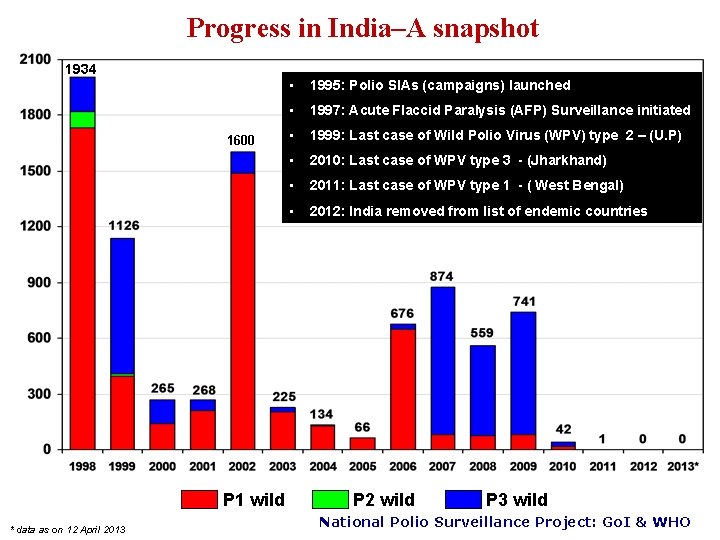
WPV cases, India 1934 No case since Jan 2011 1600 P 1 wild * data as on 12 April 2013 P 2 wild P 3 wild National Polio Surveillance Project: Go. I & WHO

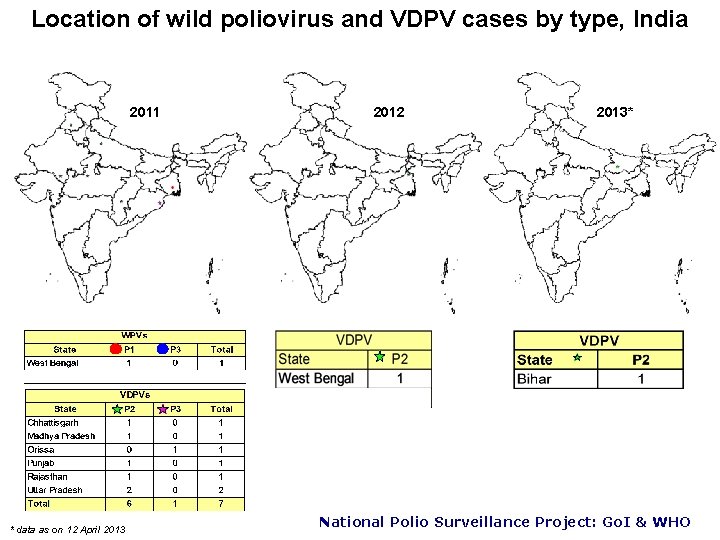
Location of wild poliovirus and VDPV cases by type, India 2011 * data as on 12 April 2013 2012 2013* National Polio Surveillance Project: Go. I & WHO

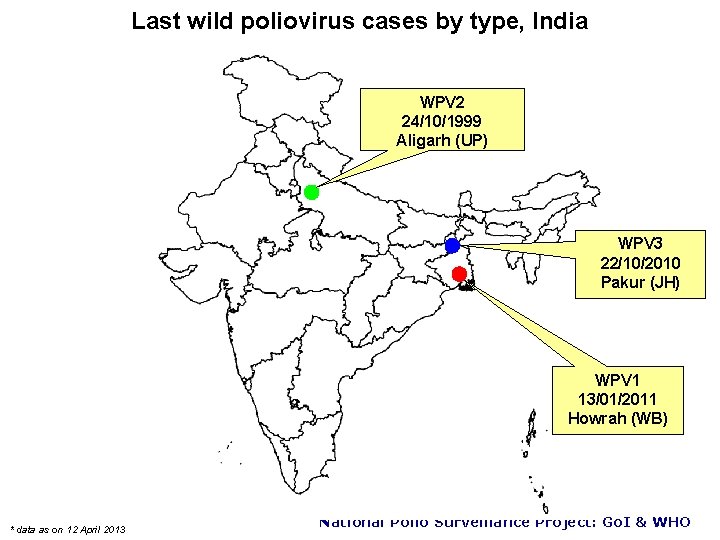
Last wild poliovirus cases by type, India WPV 2 24/10/1999 Aligarh (UP) WPV 3 22/10/2010 Pakur (JH) WPV 1 13/01/2011 Howrah (WB) * data as on 12 April 2013 National Polio Surveillance Project: Go. I & WHO

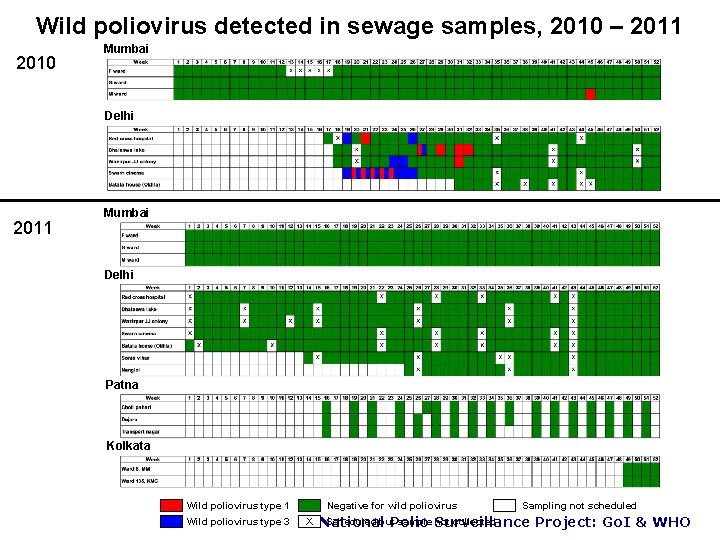
Wild poliovirus detected in sewage samples, 2010 – 2011 2010 Mumbai Delhi 2011 Mumbai Delhi Patna Kolkata Wild poliovirus type 1 Wild poliovirus type 3 Negative for wild poliovirus X Sampling not scheduled Scheduled but sample Surveillance not collected National Polio Project: Go. I & WHO

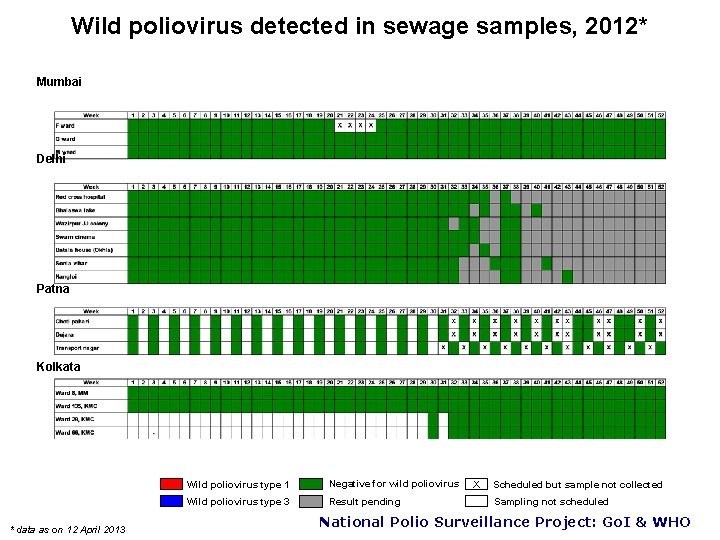
Wild poliovirus detected in sewage samples, 2012* Mumbai Delhi Patna Kolkata * data as on 12 April 2013 Wild poliovirus type 1 Negative for wild poliovirus Wild poliovirus type 3 Result pending X Scheduled but sample not collected Sampling not scheduled National Polio Surveillance Project: Go. I & WHO

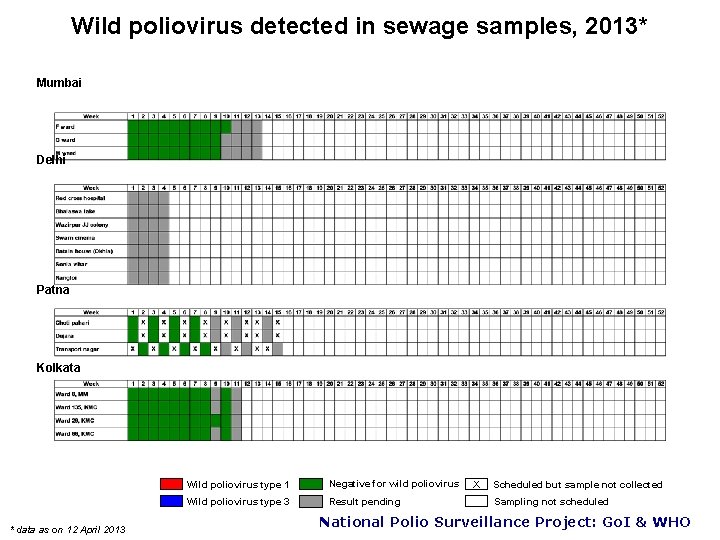
Wild poliovirus detected in sewage samples, 2013* Mumbai Delhi Patna Kolkata * data as on 12 April 2013 Wild poliovirus type 1 Negative for wild poliovirus Wild poliovirus type 3 Result pending X Scheduled but sample not collected Sampling not scheduled National Polio Surveillance Project: Go. I & WHO

Progress in India–A snapshot 1934 1600 P 1 wild * data as on 12 April 2013 • 1995: Polio SIAs (campaigns) launched • 1997: Acute Flaccid Paralysis (AFP) Surveillance initiated • 1999: Last case of Wild Polio Virus (WPV) type 2 – (U. P) • 2010: Last case of WPV type 3 - (Jharkhand) • 2011: Last case of WPV type 1 - ( West Bengal) • 2012: India removed from list of endemic countries P 2 wild P 3 wild National Polio Surveillance Project: Go. I & WHO

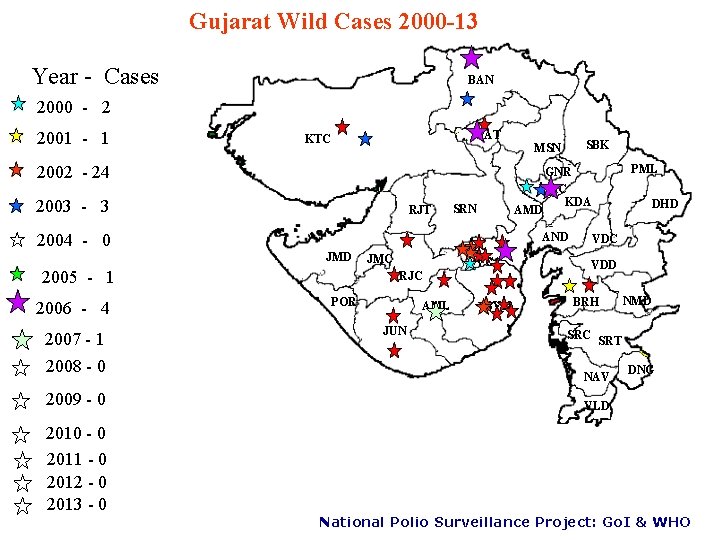
Gujarat Wild Cases 2000 -13 Year - Cases BAN 2000 - 2 2001 - 1 PAT KTC 2002 - 24 2003 - 3 RJT SRN AND JMD 2005 - 1 2007 - 1 2008 - 0 2009 - 0 2010 - 0 2011 - 0 2012 - 0 2013 - 0 JMC BVC RJC POR AML JUN PML GNR AMC KDA AMD 2004 - 0 2006 - 4 SBK MSN BVN DHD VDC VDD BRH NMD SRC SRT NAV DNG VLD National Polio Surveillance Project: Go. I & WHO

Epidemiology of Polio National Polio Surveillance Project: Go. I & WHO

Polio: Epidemiology • Reservoir of infection: Man (for every clinical case, 1000 sub clinical case (children) & 75 (adults) • Infective material: feces • Period of communicability: most infective 10 days before & after onset. Host factors • Age: 6 months to 3 years most common • Sex: 3: 1 ---male: female • Precipitating factors: fatigue, trauma, I/M injections • Immunity : OPV (life long) Environmental factors • Rainy season (Jun – Sep), overcrowding & poor sanitation National Polio Surveillance Project: Go. I & WHO

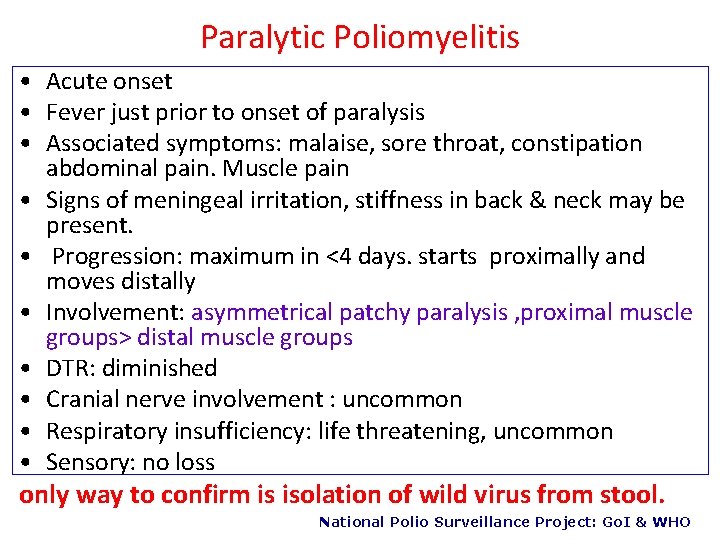
Paralytic Poliomyelitis • Acute onset • Fever just prior to onset of paralysis • Associated symptoms: malaise, sore throat, constipation abdominal pain. Muscle pain • Signs of meningeal irritation, stiffness in back & neck may be present. • Progression: maximum in <4 days. starts proximally and moves distally • Involvement: asymmetrical patchy paralysis , proximal muscle groups> distal muscle groups • DTR: diminished • Cranial nerve involvement : uncommon • Respiratory insufficiency: life threatening, uncommon • Sensory: no loss only way to confirm is isolation of wild virus from stool. National Polio Surveillance Project: Go. I & WHO

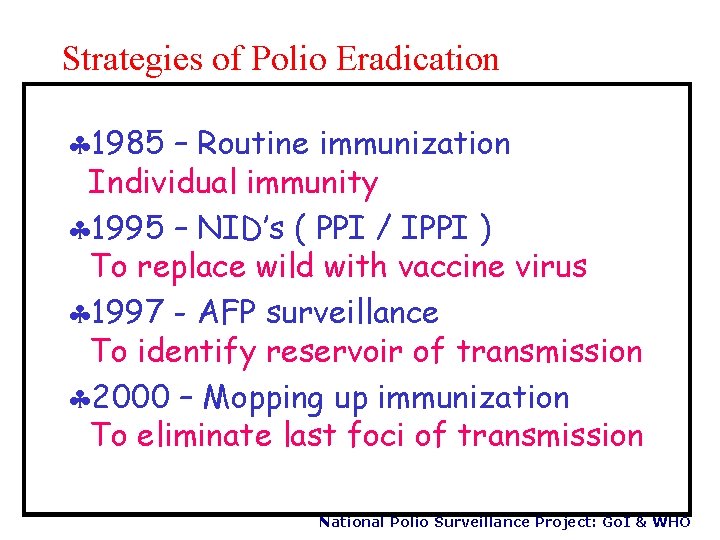
Strategies of Polio Eradication § 1985 – Routine immunization Individual immunity § 1995 – NID’s ( PPI / IPPI ) To replace wild with vaccine virus § 1997 - AFP surveillance To identify reservoir of transmission § 2000 – Mopping up immunization To eliminate last foci of transmission National Polio Surveillance Project: Go. I & WHO

AFP Surveillance National Polio Surveillance Project: Go. I & WHO

Objective of AFP surveillance Reliably detect areas where polio transmission is occurring or likely to occur National Polio Surveillance Project: Go. I & WHO

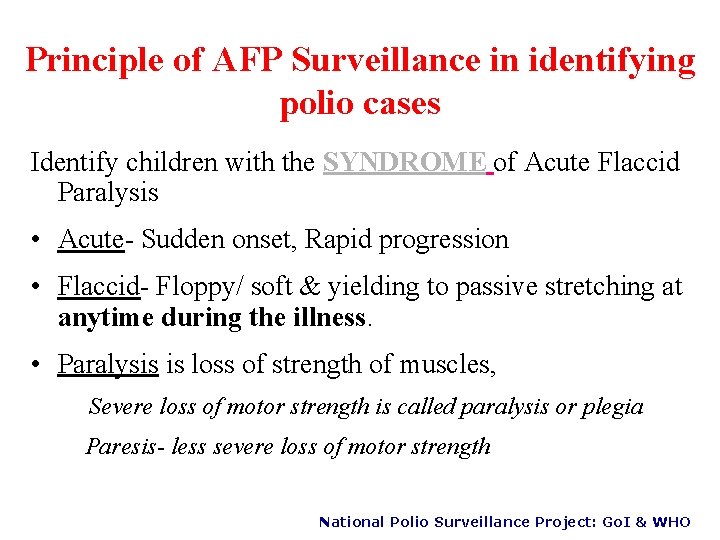
Principle of AFP Surveillance in identifying polio cases Identify children with the SYNDROME of Acute Flaccid Paralysis • Acute- Sudden onset, Rapid progression • Flaccid- Floppy/ soft & yielding to passive stretching at anytime during the illness. • Paralysis is loss of strength of muscles, Severe loss of motor strength is called paralysis or plegia Paresis- less severe loss of motor strength National Polio Surveillance Project: Go. I & WHO

Definition of AFP for surveillance purposes Sudden onset weakness & floppiness in any part of the body in a child < 15 years of age or paralysis in a person of any age in which polio is suspected. National Polio Surveillance Project: Go. I & WHO

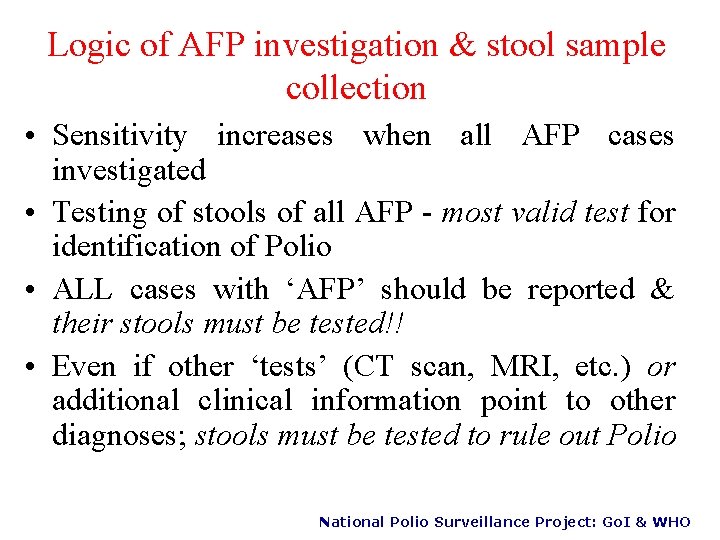
Logic of AFP investigation & stool sample collection • Sensitivity increases when all AFP cases investigated • Testing of stools of all AFP - most valid test for identification of Polio • ALL cases with ‘AFP’ should be reported & their stools must be tested!! • Even if other ‘tests’ (CT scan, MRI, etc. ) or additional clinical information point to other diagnoses; stools must be tested to rule out Polio National Polio Surveillance Project: Go. I & WHO

Reporting • All AFP cases should be reported immediately • ALL AFP cases reported within 6 months of onset of paralysis should be investigated • All reporting units, informers and other contacts should continue to report AFP cases as per existing case definition National Polio Surveillance Project: Go. I & WHO

Action when AFP is reported • FIRST – Start stool collection process • Investigate - SMO/ DIO - Confirm if AFP, if not reject case & record the same. There is only one category of cases - AFP • Allot EPID number & Report the case as AFP • CIF & LRF should be filled. • Use the revised CIF/ Linelist form. • Ensure that stools are transported to lab in cold chain • NPSU will Classify after lab result received • Give feedback to the source that the AFP reported was/ was not polio. • Maintain documentation at ALL levels. National Polio Surveillance Project: Go. I & WHO

Therefore… The basic system of AFP surveillance remains unchanged • To enhance sensitivity, all cases of acute flaccid paralysis should be reported & investigated • Borderline cases should be included & stool specimens tested National Polio Surveillance Project: Go. I & WHO

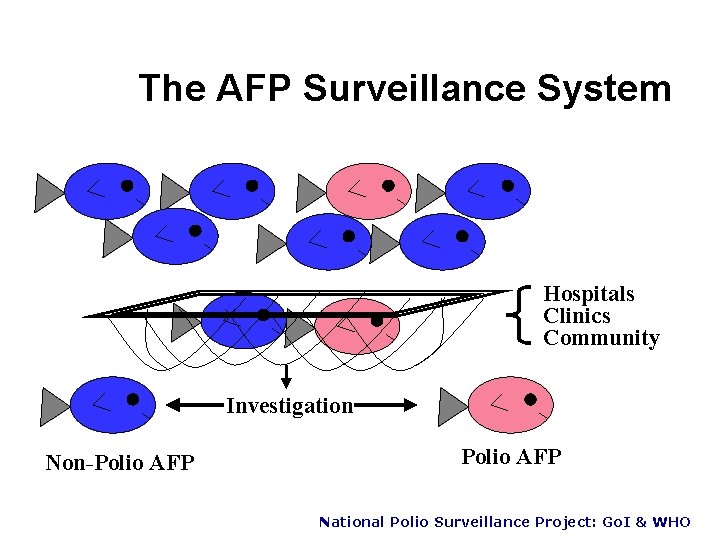
The AFP Surveillance System Hospitals Clinics Community Investigation Non-Polio AFP National Polio Surveillance Project: Go. I & WHO

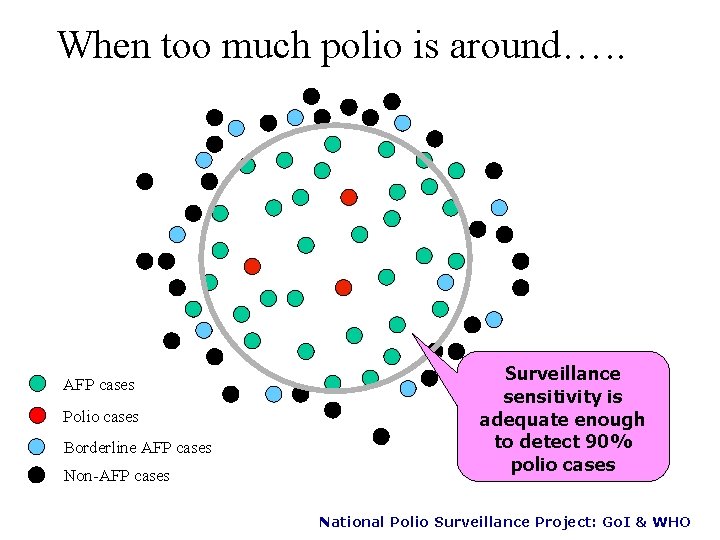
When too much polio is around…. . AFP cases Polio cases Borderline AFP cases Non-AFP cases Surveillance sensitivity is adequate enough to detect 90% polio cases National Polio Surveillance Project: Go. I & WHO

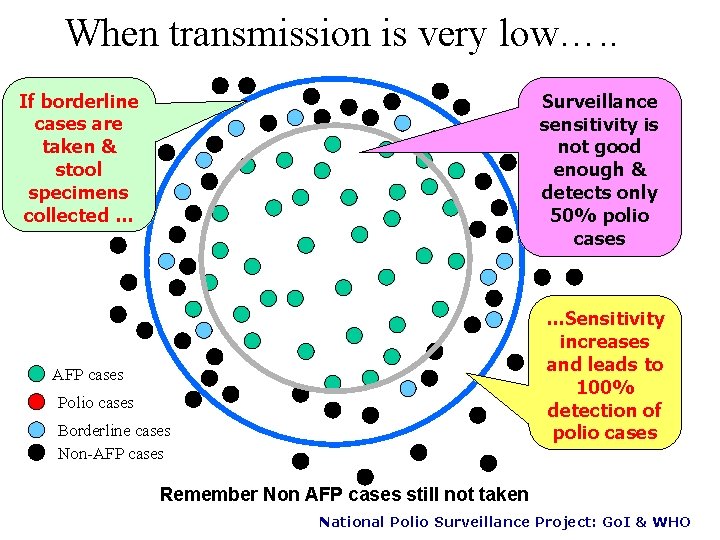
When transmission is very low…. . If borderline cases are taken & stool specimens collected … Surveillance sensitivity is not good enough & detects only 50% polio cases …Sensitivity increases and leads to 100% detection of polio cases AFP cases Polio cases Borderline cases Non-AFP cases Remember Non AFP cases still not taken National Polio Surveillance Project: Go. I & WHO

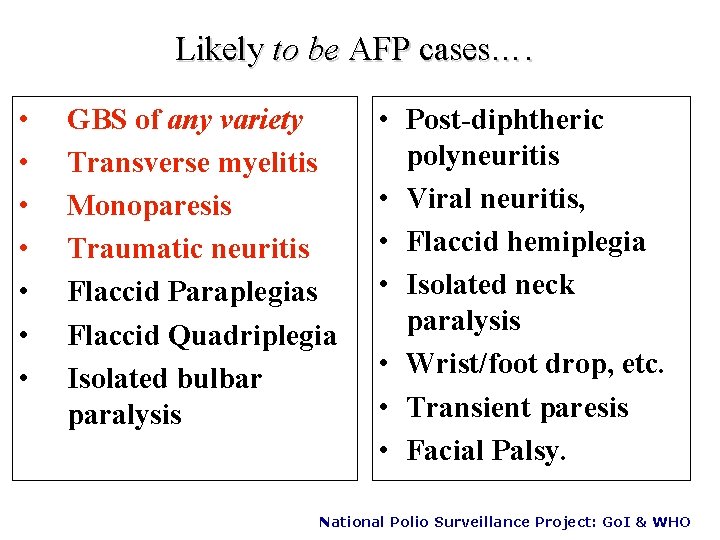
Likely to be AFP cases…. • • GBS of any variety Transverse myelitis Monoparesis Traumatic neuritis Flaccid Paraplegias Flaccid Quadriplegia Isolated bulbar paralysis • Post-diphtheric polyneuritis • Viral neuritis, • Flaccid hemiplegia • Isolated neck paralysis • Wrist/foot drop, etc. • Transient paresis • Facial Palsy. National Polio Surveillance Project: Go. I & WHO

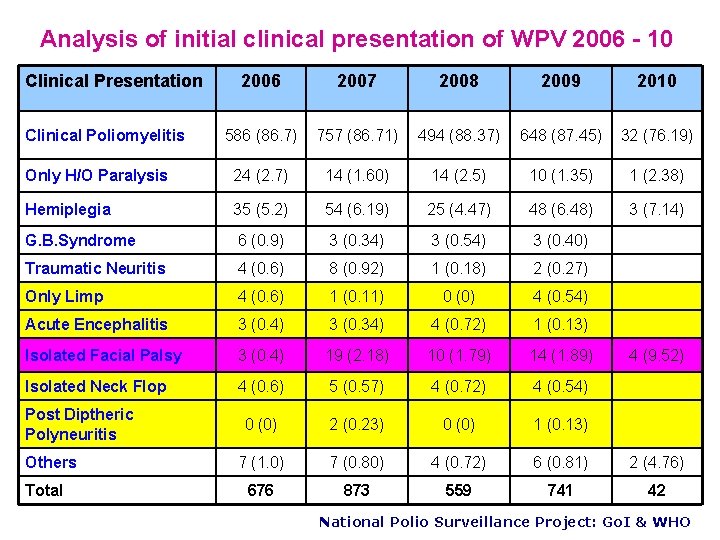
Analysis of initial clinical presentation of WPV 2006 - 10 Clinical Presentation 2006 2007 2008 2009 2010 586 (86. 7) 757 (86. 71) 494 (88. 37) 648 (87. 45) 32 (76. 19) Only H/O Paralysis 24 (2. 7) 14 (1. 60) 14 (2. 5) 10 (1. 35) 1 (2. 38) Hemiplegia 35 (5. 2) 54 (6. 19) 25 (4. 47) 48 (6. 48) 3 (7. 14) G. B. Syndrome 6 (0. 9) 3 (0. 34) 3 (0. 54) 3 (0. 40) Traumatic Neuritis 4 (0. 6) 8 (0. 92) 1 (0. 18) 2 (0. 27) Only Limp 4 (0. 6) 1 (0. 11) 0 (0) 4 (0. 54) Acute Encephalitis 3 (0. 4) 3 (0. 34) 4 (0. 72) 1 (0. 13) Isolated Facial Palsy 3 (0. 4) 19 (2. 18) 10 (1. 79) 14 (1. 89) Isolated Neck Flop 4 (0. 6) 5 (0. 57) 4 (0. 72) 4 (0. 54) 0 (0) 2 (0. 23) 0 (0) 1 (0. 13) 7 (1. 0) 7 (0. 80) 4 (0. 72) 6 (0. 81) 2 (4. 76) 676 873 559 741 42 Clinical Poliomyelitis Post Diptheric Polyneuritis Others Total 4 (9. 52) National Polio Surveillance Project: Go. I & WHO

STOOL COLLECTION, STORAGE , TRANSPORT. • Adequate Stool. – 2 Specimens, 24 Hours Apart. – 8 gms. – Within 14 Days of Paralysis Onset & with proper Cold Chain • Procedure. – Errors. – Storage(Delayed Second Sample) • Cold Chain. • Rectal Tube. • Transport. (PHN & HA) • Death of AFP Case. ( Spinal Cord , Intestinal Content) National Polio Surveillance Project: Go. I & WHO

GOLD STANDARD FOR AFP SURVEILLANCE • Non – Polio AFP Rate > 2. 0 • Adequate Stool Samples > 80% • Timeliness of Reporting > 80% National Polio Surveillance Project: Go. I & WHO

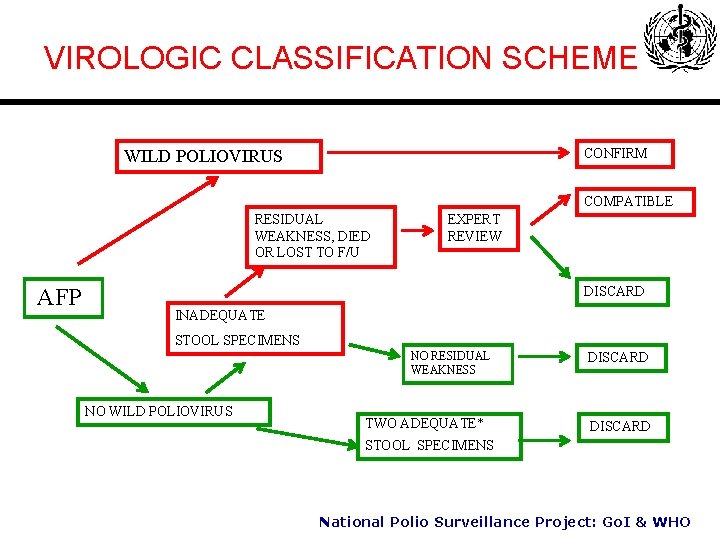
VIROLOGIC CLASSIFICATION SCHEME CONFIRM WILD POLIOVIRUS COMPATIBLE RESIDUAL WEAKNESS, DIED OR LOST TO F/U AFP EXPERT REVIEW DISCARD INADEQUATE STOOL SPECIMENS NO RESIDUAL WEAKNESS NO WILD POLIOVIRUS TWO ADEQUATE* DISCARD STOOL SPECIMENS National Polio Surveillance Project: Go. I & WHO

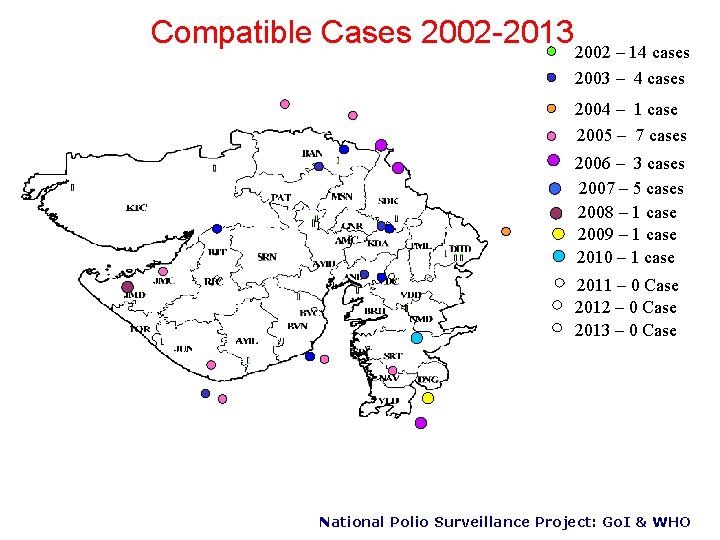
Compatible Cases 2002 -2013 2002 – 14 cases 2003 – 4 cases 2004 – 1 case 2005 – 7 cases 2006 – 3 cases 2007 – 5 cases 2008 – 1 case 2009 – 1 case 2010 – 1 case 2011 – 0 Case 2012 – 0 Case 2013 – 0 Case National Polio Surveillance Project: Go. I & WHO

HOT CASE • A case of AFP with any of the following set of conditions Ø Age < 5 year plus H/O fever at onset plus asymmetrical proximal paralysis. Ø Age < 5 year with rapidly progressive paralysis leading to bulbar involvement (cranial nerves affected) & death. Ø Any case which in the opinion of SMO/DIO looks like polio. National Polio Surveillance Project: Go. I & WHO

CONTACT SAMPLES To be considered for cases fulfilling criteria like Hot cases, but adequate samples from case could not be taken National Polio Surveillance Project: Go. I & WHO

Supplementary Immunization Activities: NIDs/ SNIDs National Polio Surveillance Project: Go. I & WHO

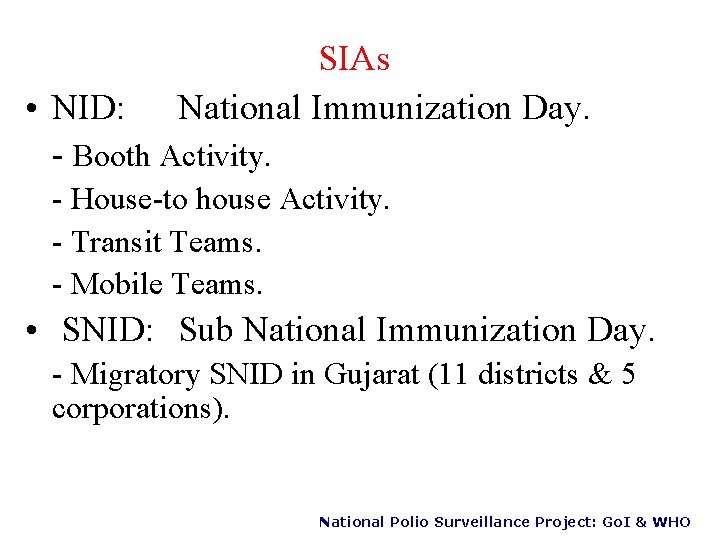
SIAs National Immunization Day. • NID: - Booth Activity. - House-to house Activity. - Transit Teams. - Mobile Teams. • SNID: Sub National Immunization Day. - Migratory SNID in Gujarat (11 districts & 5 corporations). National Polio Surveillance Project: Go. I & WHO

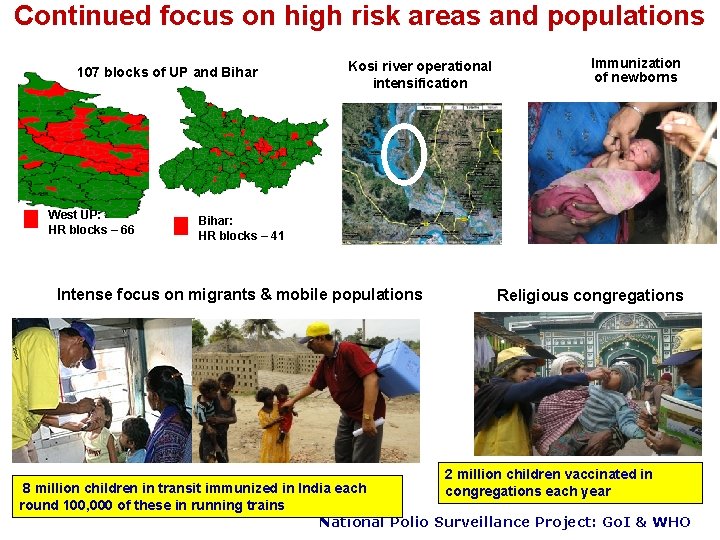
Continued focus on high risk areas and populations 107 blocks of UP and Bihar West UP: HR blocks – 66 Kosi river operational intensification Immunization of newborns Bihar: HR blocks – 41 Intense focus on migrants & mobile populations Religious congregations 2 million children vaccinated in congregations each year 8 million children in transit immunized in India each round 100, 000 of these in running trains National Polio Surveillance Project: Go. I & WHO

Certification of polio eradication National Polio Surveillance Project: Go. I & WHO

Background • Certification is done for WHO Regions; not for individual countries • WHO Regions certified polio free: – Americas 1994 – Western Pacific 2000 – Europe 2002 • Certification of a region is considered only when – All countries in the area demonstrate ØAbsence of WPV transmission for at least 3 consecutive years ØPresence of certification standard surveillance ØGlobal action plan for laboratory containment of WPV National Polio Surveillance Project: Go. I & WHO

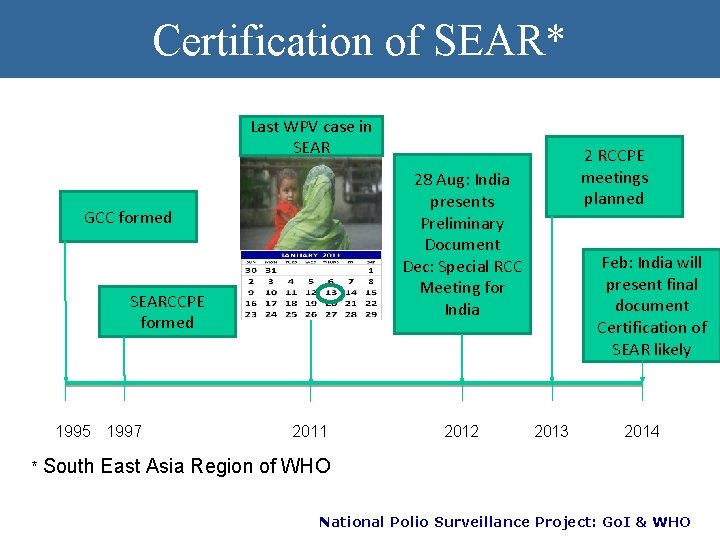
Certification of SEAR* Last WPV case in SEAR 28 Aug: India presents Preliminary Document Dec: Special RCC Meeting for India GCC formed SEARCCPE formed 1995 1997 * South 2 RCCPE meetings planned 2011 2012 Feb: India will present final document Certification of SEAR likely 2013 2014 East Asia Region of WHO National Polio Surveillance Project: Go. I & WHO

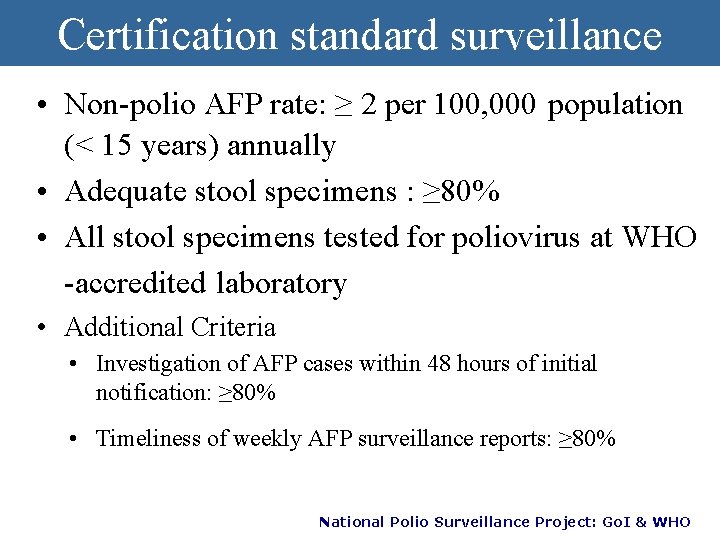
Certification standard surveillance • Non-polio AFP rate: ≥ 2 per 100, 000 population (< 15 years) annually • Adequate stool specimens : ≥ 80% • All stool specimens tested for poliovirus at WHO -accredited laboratory • Additional Criteria • Investigation of AFP cases within 48 hours of initial notification: ≥ 80% • Timeliness of weekly AFP surveillance reports: ≥ 80% National Polio Surveillance Project: Go. I & WHO

National Certification Committee for Poliomyelitis Eradication (NCCPE) • Established in 1998 to • Examine, assess & verify data collected by govt. • Field visits to review evidence of interruption of poliovirus transmission in the country • Independent judgment of polio status • Present country report to RCCPE* Regional Certification Commission for Poliomyelitis Eradication * National Polio Surveillance Project: Go. I & WHO

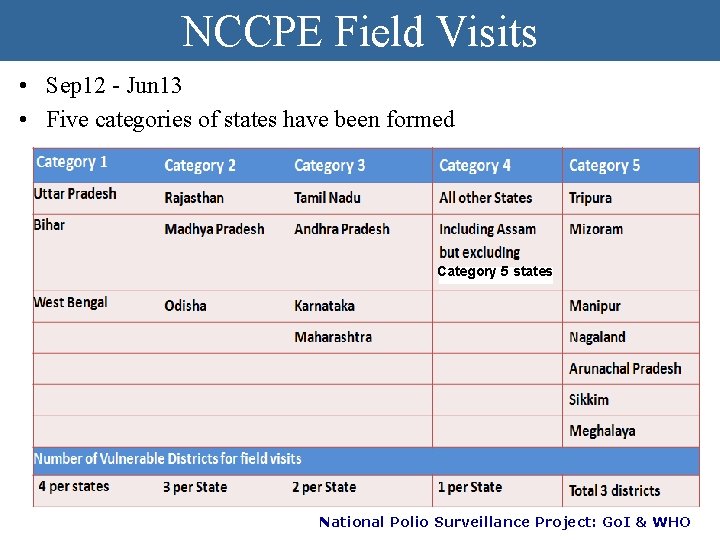
NCCPE Field Visits • Sep 12 - Jun 13 • Five categories of states have been formed Category 5 states National Polio Surveillance Project: Go. I & WHO

Laboratory Containment • Union Health Ministry already issued letter in this regard to all the States (dated 14 th Feb 2013). • National Task Force for Lab Containment of WPV formed, Health Secretary (GOI) Chairman. • To identify Labs, likely to store WPV – by Dec 2013. National Polio Surveillance Project: Go. I & WHO

Polio Endgame Strategy National Polio Surveillance Project: Go. I & WHO

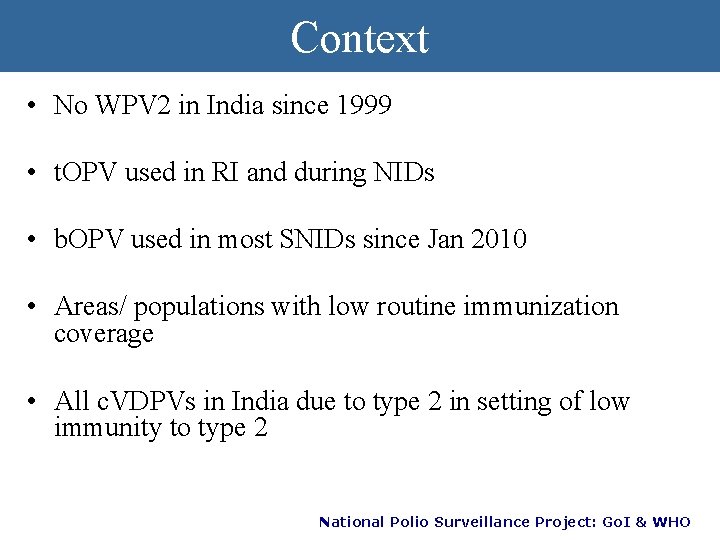
Context • No WPV 2 in India since 1999 • t. OPV used in RI and during NIDs • b. OPV used in most SNIDs since Jan 2010 • Areas/ populations with low routine immunization coverage • All c. VDPVs in India due to type 2 in setting of low immunity to type 2 National Polio Surveillance Project: Go. I & WHO

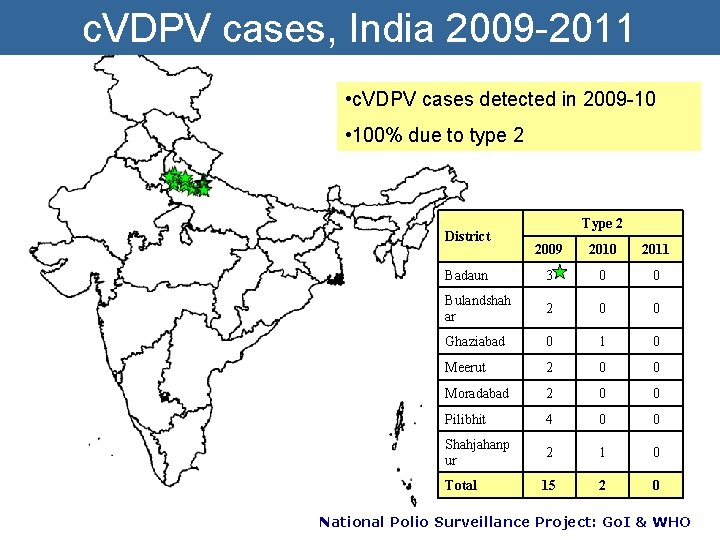
c. VDPV cases, India 2009 -2011 • c. VDPV cases detected in 2009 -10 • 100% due to type 2 District Type 2 2009 2010 2011 Badaun 3 0 0 Bulandshah ar 2 0 0 Ghaziabad 0 1 0 Meerut 2 0 0 Moradabad 2 0 0 Pilibhit 4 0 0 Shahjahanp ur 2 1 0 Total 15 2 0 National Polio Surveillance Project: Go. I & WHO

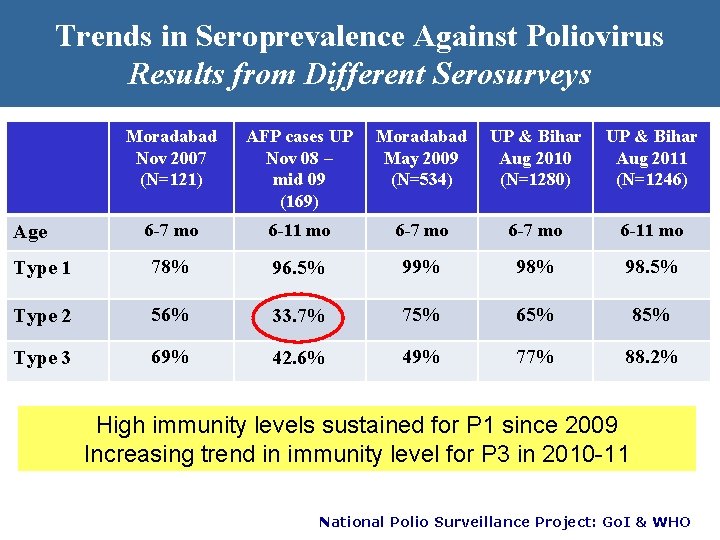
Trends in Seroprevalence Against Poliovirus Results from Different Serosurveys Moradabad Nov 2007 (N=121) AFP cases UP Nov 08 – mid 09 (169) Moradabad May 2009 (N=534) UP & Bihar Aug 2010 (N=1280) UP & Bihar Aug 2011 (N=1246) 6 -7 mo 6 -11 mo Type 1 78% 96. 5% 99% 98. 5% Type 2 56% 33. 7% 75% 65% 85% Type 3 69% 42. 6% 49% 77% 88. 2% Age High immunity levels sustained for P 1 since 2009 Increasing trend in immunity level for P 3 in 2010 -11 National Polio Surveillance Project: Go. I & WHO

Managing the risk of VDPVs National Polio Surveillance Project: Go. I & WHO

Preparing for the polio endgame • A t. OPV- b. OPV switch globally (~2014/2015) • Use of IPV in conjunction with OPV (? ) • Eventual cessation of all OPV use globally at some point in the future (e. g. 2017 -18 period). • Support research activities to generate evidence to guide decision making National Polio Surveillance Project: Go. I & WHO

Pre-switch boosting of type 2 immunity • Switch soon after t. OPV NIDs • Improve RI, particularly DTP 3 and OPV 3 coverage • Adding a dose of IPV in RI for infants prior to switch National Polio Surveillance Project: Go. I & WHO

Conclusions • India can be in a position to move ahead with polio endgame strategy • Careful planning and consideration of risks required before implementation • Earliest possible timing for t. OPV-b. OPV switch: Qtr. 1 2014 • Lessons from t. OPV-b. OPV switch significant for subsequent withdrawal of all OPV from programme National Polio Surveillance Project: Go. I & WHO

Thank you National Polio Surveillance Project: Go. I & WHO