Mumps and Mumps Vaccine Epidemiology and Prevention of













- Slides: 19

Mumps and Mumps Vaccine Epidemiology and Prevention of Vaccine. Preventable Diseases National Immunization Program Centers for Disease Control and Prevention Revised March 2002

Mumps • Acute viral illness • Parotitis and orchitis described by Hippocrates in 5 th century B. C. • Viral etiology described by Johnson and Goodpasture in 1934 • Frequent cause of outbreaks among military personnel in prevaccine era

Mumps Virus • Paramyxovirus • RNA virus • One antigenic type • Rapidly inactivated by chemical agents, heat and ultraviolet light

Mumps Pathogenesis • Respiratory transmission of virus • Replication in nasopharynx and regional lymph nodes • Viremia 12 -25 days after exposure with spread to tissues • Multiple tissues infected during viremia

Mumps Clinical Features • Incubation period 14 -18 days • Nonspecific prodrome of low-grade fever, headache, malaise, myalgias • Parotitis in 30%-40% • Up to 20% of infections asymptomatic • May present as lower respiratory illness, particularly in preschool-aged children

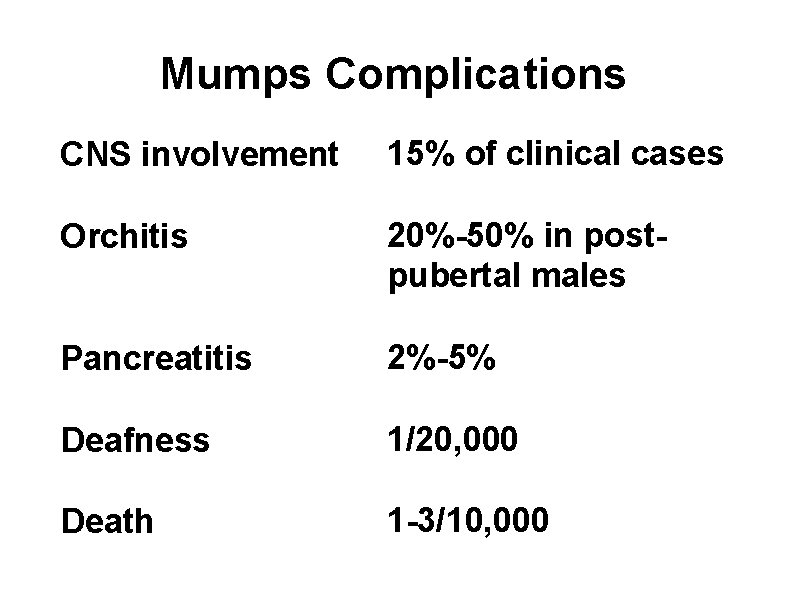
Mumps Complications CNS involvement 15% of clinical cases Orchitis 20%-50% in postpubertal males Pancreatitis 2%-5% Deafness 1/20, 000 Death 1 -3/10, 000

Mumps Laboratory Diagnosis • Isolation of mumps virus • Serologic testing –positive Ig. M antibody –significant increase in Ig. G antibody between acute and convalescent specimens

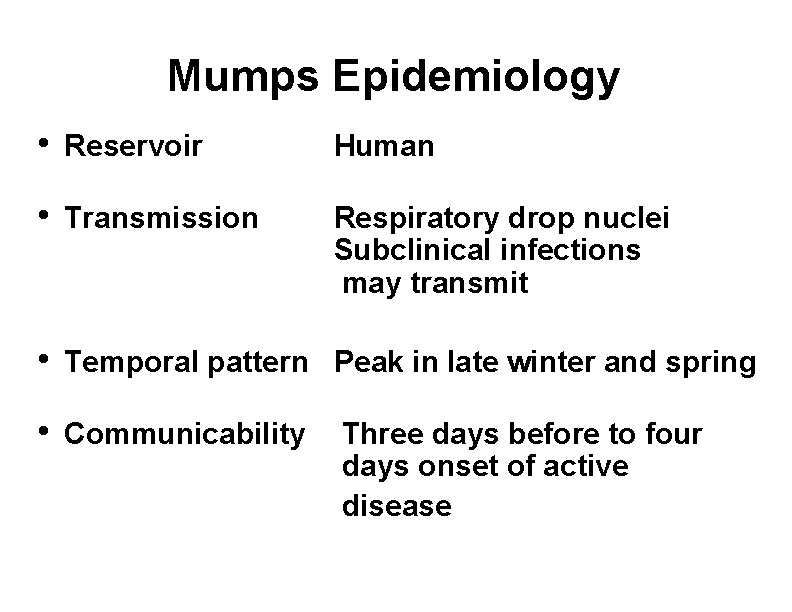
Mumps Epidemiology • Reservoir Human • Transmission Respiratory drop nuclei Subclinical infections may transmit • Temporal pattern Peak in late winter and spring • Communicability Three days before to four days onset of active disease

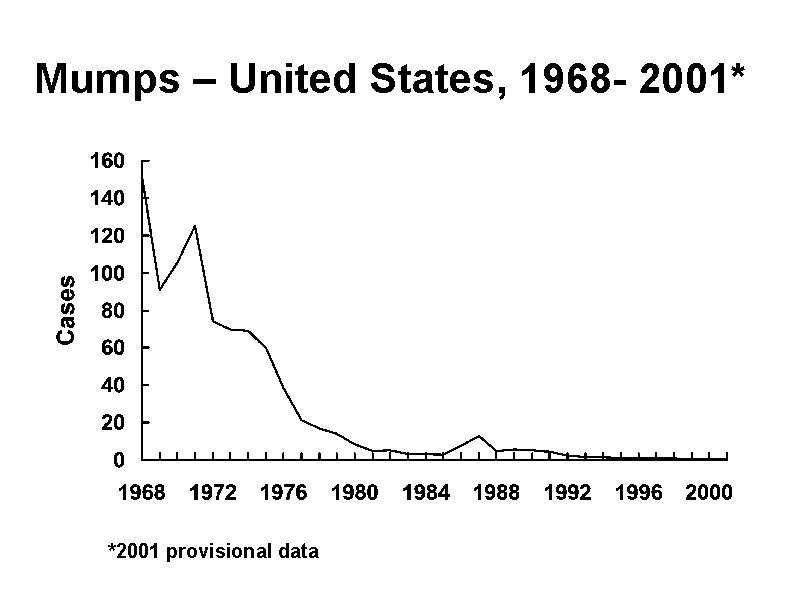
Mumps – United States, 1968 - 2001* *2001 provisional data

Mumps – United States, 1980 -2001* *2001 provisional data

Mumps - United States, 1980 -2000 Age Distribution of Reported Cases

Mumps Clinical Case Definition • Acute onset of unilateral or bilateral swelling of parotid or salivary gland lasting >2 days without other apparent cause.

Mumps Vaccine • Composition Live virus (Jeryl Lynn strain) • Efficacy 95% (Range, 90%-97%) • Duration of Immunity Lifelong • Schedule 1 Dose • Should be administered with measles and rubella (MMR)

Mumps (MMR) Vaccine Indications • All infants >12 months of age • Susceptible adolescents and adults without documented evidence of immunity

Mumps Immunity • Born before 1957 • Documentation of physiciandiagnosed mumps • Serologic evidence of mumps immunity • Documentation of adequate vaccination

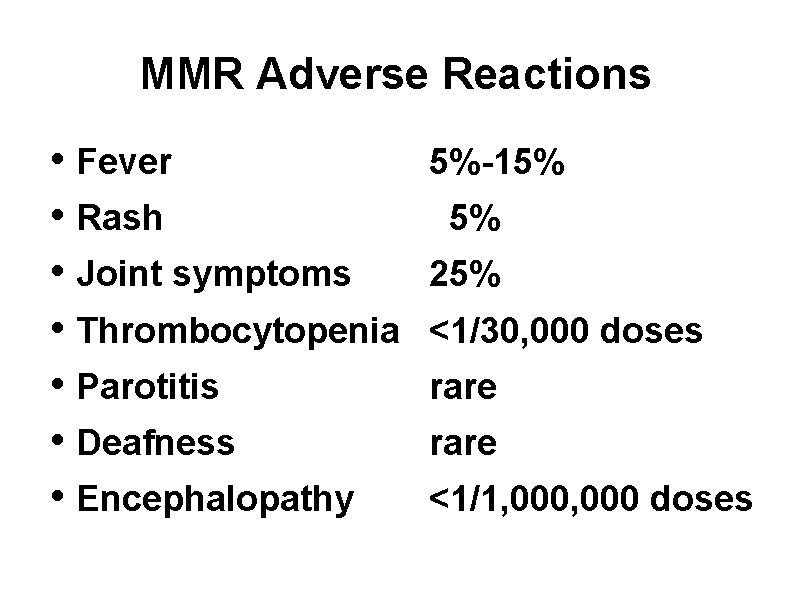
MMR Adverse Reactions • Fever • Rash • Joint symptoms • Thrombocytopenia • Parotitis • Deafness • Encephalopathy 5%-15% 5% 25% <1/30, 000 doses rare <1/1, 000 doses

MMR Vaccine Contraindications and Precautions • Severe allergic reaction to prior dose or vaccine component • Pregnancy • Immunosuppression • Moderate or severe acute illness • Recent blood product

Measles and Mumps Vaccines and Egg Allergy • Measles and mumps viruses grown in chick embryo fibroblast culture • Studies have demonstrated safety of MMR in egg allergic children • Vaccinate without testing

National Immunization Program • Hotline 800. 232. 2522 • Email nipinfo@cdc. gov • Website www. cdc. gov/nip
 Primary prevention secondary prevention tertiary prevention
Primary prevention secondary prevention tertiary prevention Mumps medicine
Mumps medicine Mumps virus
Mumps virus Pathophysiology of mumps
Pathophysiology of mumps Mumps db
Mumps db Nursing care plan of mumps
Nursing care plan of mumps Mumps virus
Mumps virus Thesourceagents
Thesourceagents Advantages and disadvantages of nutritional epidemiology
Advantages and disadvantages of nutritional epidemiology Difference between descriptive and analytical epidemiology
Difference between descriptive and analytical epidemiology Descriptive vs analytic epidemiology examples
Descriptive vs analytic epidemiology examples Cbic recertification
Cbic recertification Person place time epidemiology
Person place time epidemiology Ukuran asosiasi epidemiologi
Ukuran asosiasi epidemiologi Logistic regression epidemiology
Logistic regression epidemiology Point and period prevalence
Point and period prevalence Classification of epidemiological studies
Classification of epidemiological studies Attack rate epidemiology formula
Attack rate epidemiology formula Bibliography of epidemiology
Bibliography of epidemiology Recall bias
Recall bias