PlasmaSurface Interactions at a Spinning Wall Vincent M

- Slides: 32

Plasma-Surface Interactions at a “Spinning Wall” Vincent M. Donnelly Department of Chemical Engineering University of Houston, Texas Students: Joydeep Guha (now at Lam Research), Rohit Khare Postdocs: Peter Kurunczi (now at Varian), Luc Stafford (now at Univ. Montreal) Visiting Professor Yi-Kang Pu (Tsinghua University, Beijing, China) Supported by the National Science Foundation, the Department of Energy, the American Chemical Society’s Petroleum Research Fund, the University of Houston, and Lam Research Corp.

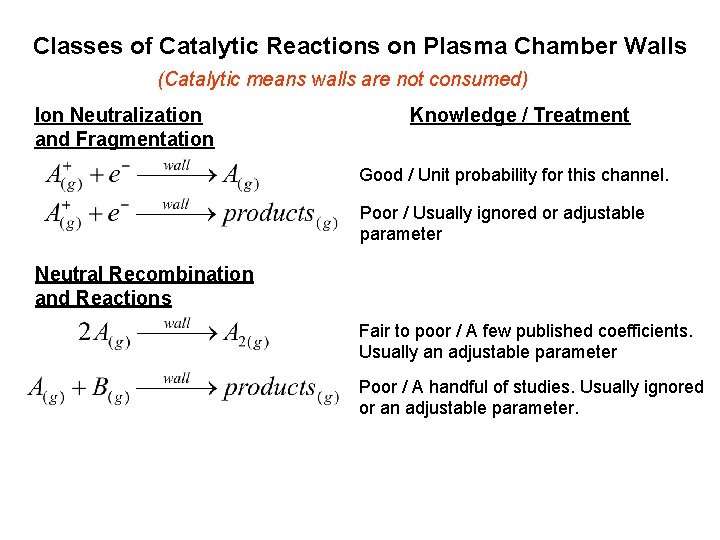
Classes of Catalytic Reactions on Plasma Chamber Walls (Catalytic means walls are not consumed) Ion Neutralization and Fragmentation Knowledge / Treatment Good / Unit probability for this channel. Poor / Usually ignored or adjustable parameter Neutral Recombination and Reactions Fair to poor / A few published coefficients. Usually an adjustable parameter Poor / A handful of studies. Usually ignored or an adjustable parameter.

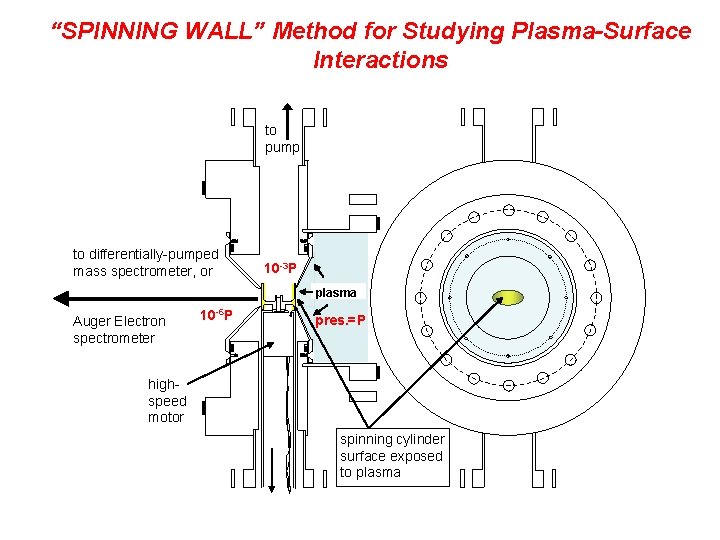
“SPINNING WALL” Method for Studying Plasma-Surface Interactions to pump to differentially-pumped mass spectrometer, or 10 -3 P plasma Auger Electron spectrometer 10 -6 P pres. =P highspeed motor spinning cylinder surface exposed to plasma

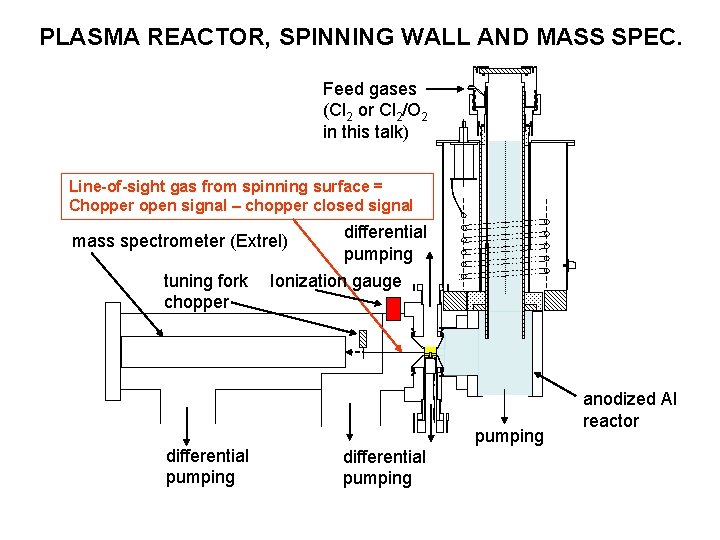
PLASMA REACTOR, SPINNING WALL AND MASS SPEC. Feed gases (Cl 2 or Cl 2/O 2 in this talk) Line-of-sight gas from spinning surface = Chopper open signal – chopper closed signal differential pumping Ionization gauge mass spectrometer (Extrel) tuning fork chopper differential pumping anodized Al reactor

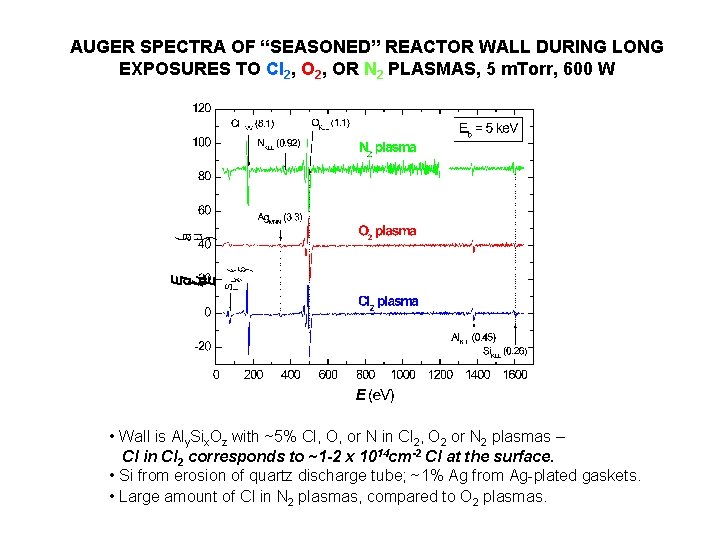
AUGER SPECTRA OF “SEASONED” REACTOR WALL DURING LONG EXPOSURES TO Cl 2, OR N 2 PLASMAS, 5 m. Torr, 600 W • Wall is Aly. Six. Oz with ~5% Cl, O, or N in Cl 2, O 2 or N 2 plasmas – Cl in Cl 2 corresponds to ~1 -2 x 1014 cm-2 Cl at the surface. • Si from erosion of quartz discharge tube; ~1% Ag from Ag-plated gaskets. • Large amount of Cl in N 2 plasmas, compared to O 2 plasmas.

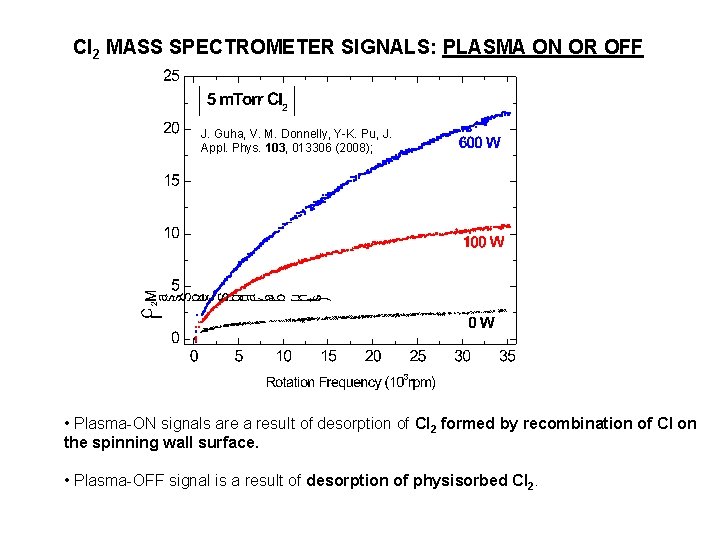
Cl 2 MASS SPECTROMETER SIGNALS: PLASMA ON OR OFF J. Guha, V. M. Donnelly, Y-K. Pu, J. Appl. Phys. 103, 013306 (2008); • Plasma-ON signals are a result of desorption of Cl 2 formed by recombination of Cl on the spinning wall surface. • Plasma-OFF signal is a result of desorption of physisorbed Cl 2.

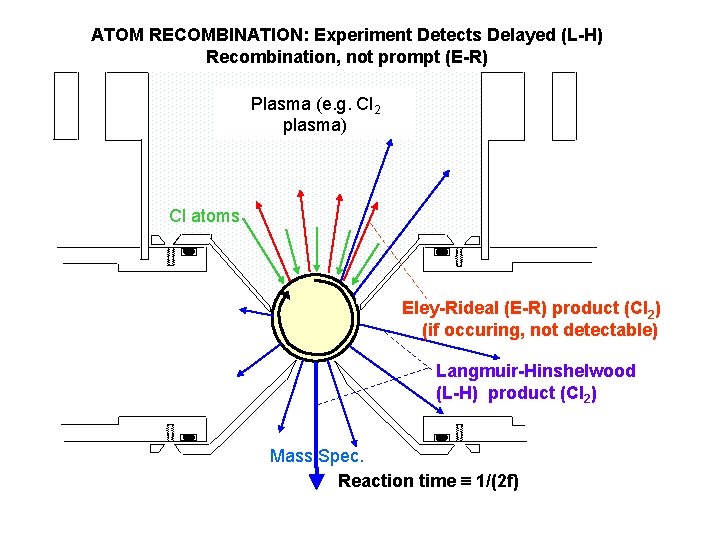
ATOM RECOMBINATION: Experiment Detects Delayed (L-H) Recombination, not prompt (E-R) Plasma (e. g. Cl 2 plasma) Cl atoms Eley-Rideal (E-R) product (Cl 2) (if occuring, not detectable) Langmuir-Hinshelwood (L-H) product (Cl 2) Mass Spec. Reaction time 1/(2 f)

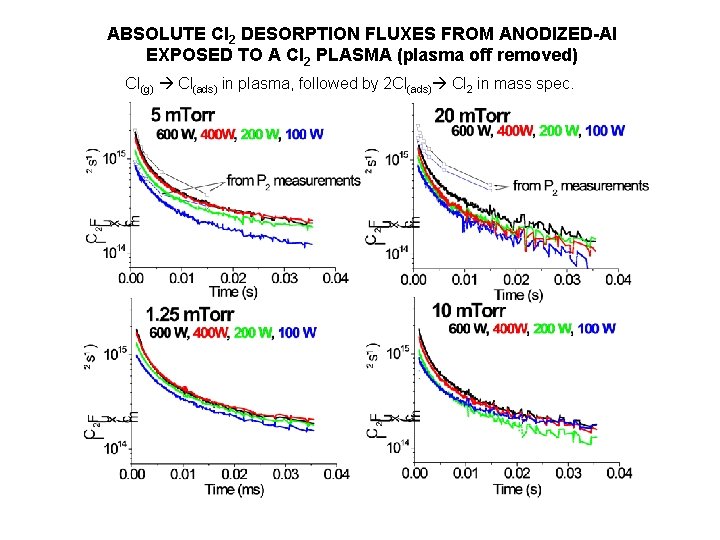
ABSOLUTE Cl 2 DESORPTION FLUXES FROM ANODIZED-Al EXPOSED TO A Cl 2 PLASMA (plasma off removed) Cl(g) Cl(ads) in plasma, followed by 2 Cl(ads) Cl 2 in mass spec.

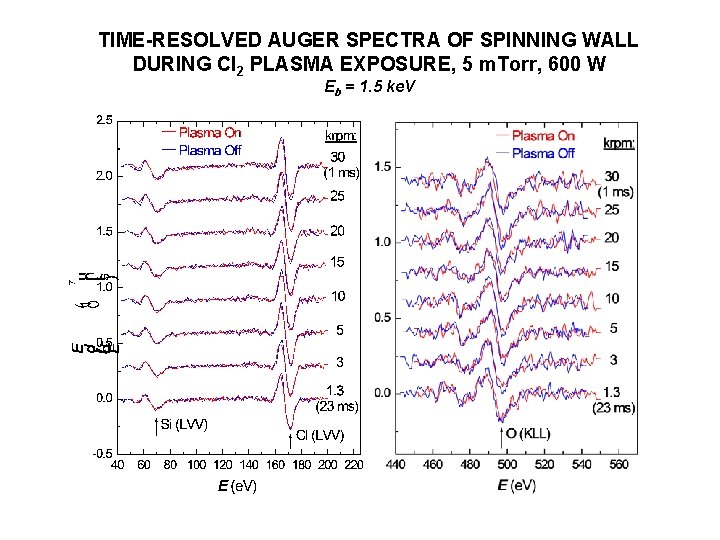
TIME-RESOLVED AUGER SPECTRA OF SPINNING WALL DURING Cl 2 PLASMA EXPOSURE, 5 m. Torr, 600 W Eb = 1. 5 ke. V

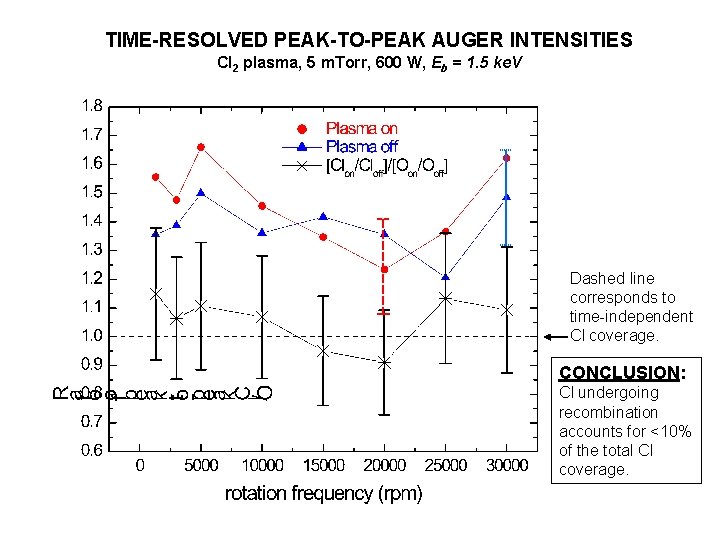
TIME-RESOLVED PEAK-TO-PEAK AUGER INTENSITIES Cl 2 plasma, 5 m. Torr, 600 W, Eb = 1. 5 ke. V Dashed line corresponds to time-independent Cl coverage. CONCLUSION: Cl undergoing recombination accounts for <10% of the total Cl coverage.

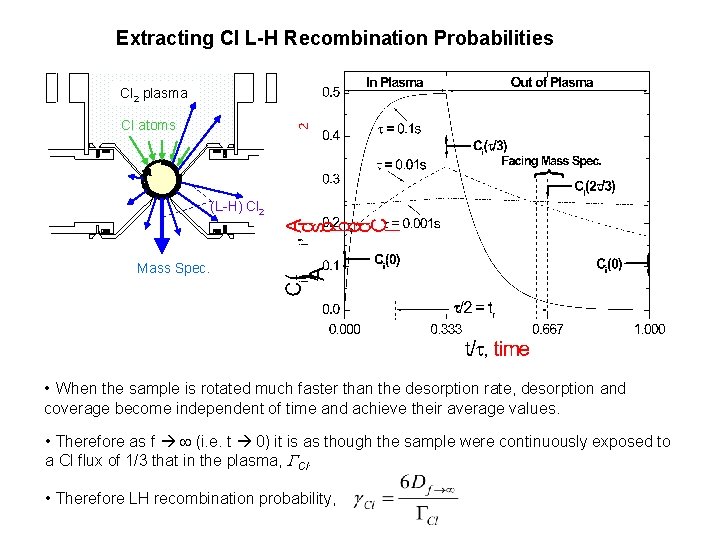
Extracting Cl L-H Recombination Probabilities Cl 2 plasma Cl atoms (L-H) Cl 2 Mass Spec. • When the sample is rotated much faster than the desorption rate, desorption and coverage become independent of time and achieve their average values. • Therefore as f (i. e. t 0) it is as though the sample were continuously exposed to a Cl flux of 1/3 that in the plasma, Cl. • Therefore LH recombination probability,

Cl Atom LH Recombination Probabilities on Anodized Al as a Function of Cl Flux and Total Pressure • Cl is small and appears to both increase and decrease with increasing Cl flux

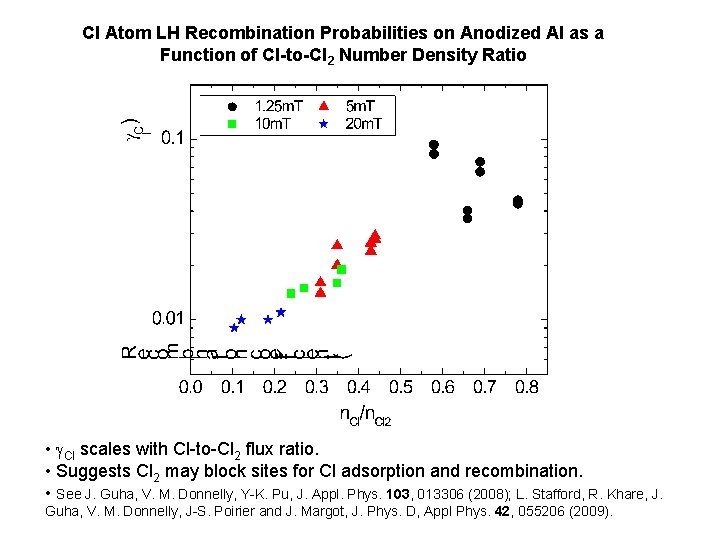
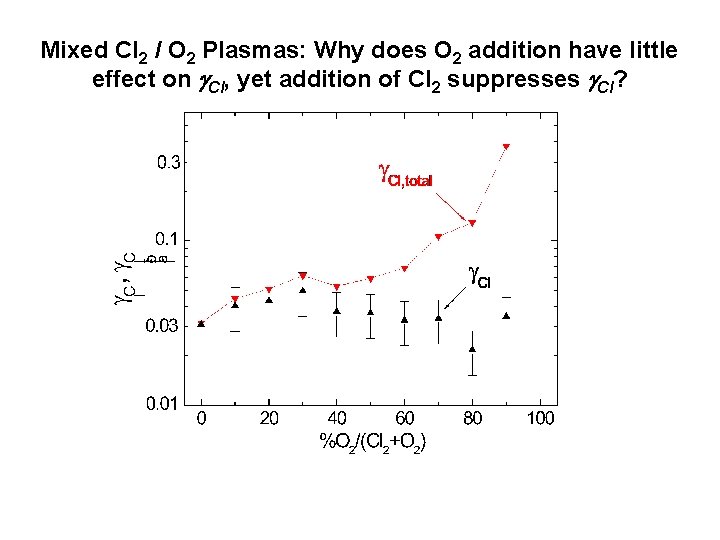
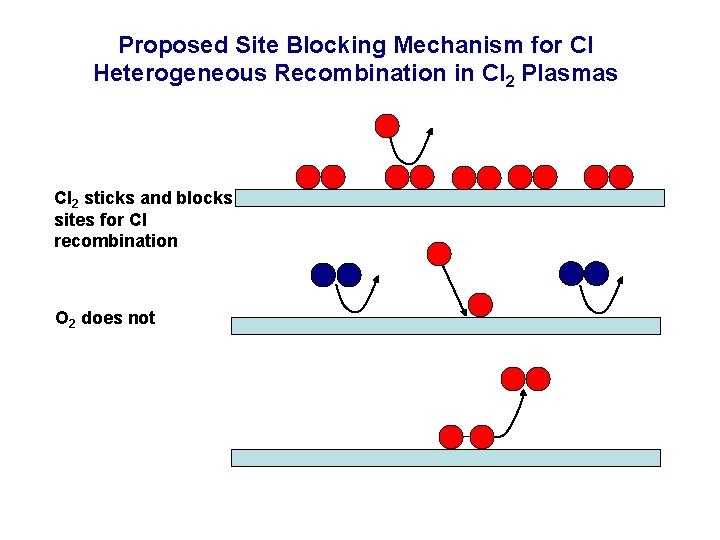
Cl Atom LH Recombination Probabilities on Anodized Al as a Function of Cl-to-Cl 2 Number Density Ratio • Cl scales with Cl-to-Cl 2 flux ratio. • Suggests Cl 2 may block sites for Cl adsorption and recombination. • See J. Guha, V. M. Donnelly, Y-K. Pu, J. Appl. Phys. 103, 013306 (2008); L. Stafford, R. Khare, J. Guha, V. M. Donnelly, J-S. Poirier and J. Margot, J. Phys. D, Appl Phys. 42, 055206 (2009).

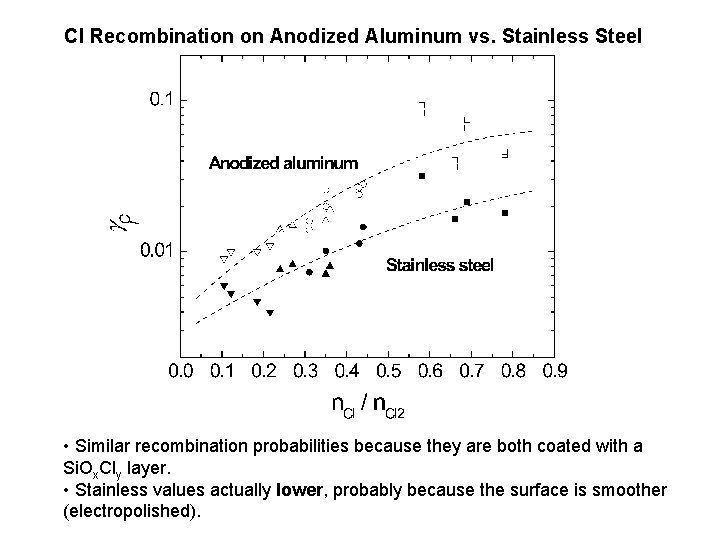
Cl Recombination on Anodized Aluminum vs. Stainless Steel • Similar recombination probabilities because they are both coated with a Si. Ox. Cly layer. • Stainless values actually lower, probably because the surface is smoother (electropolished).

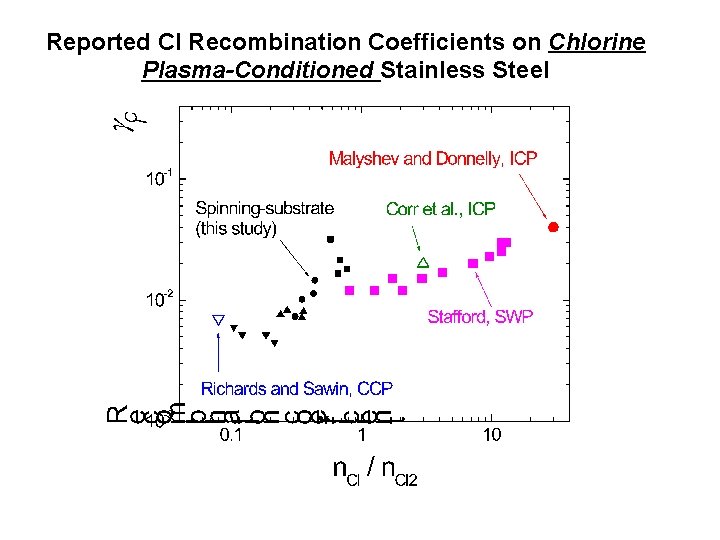
Reported Cl Recombination Coefficients on Chlorine Plasma-Conditioned Stainless Steel

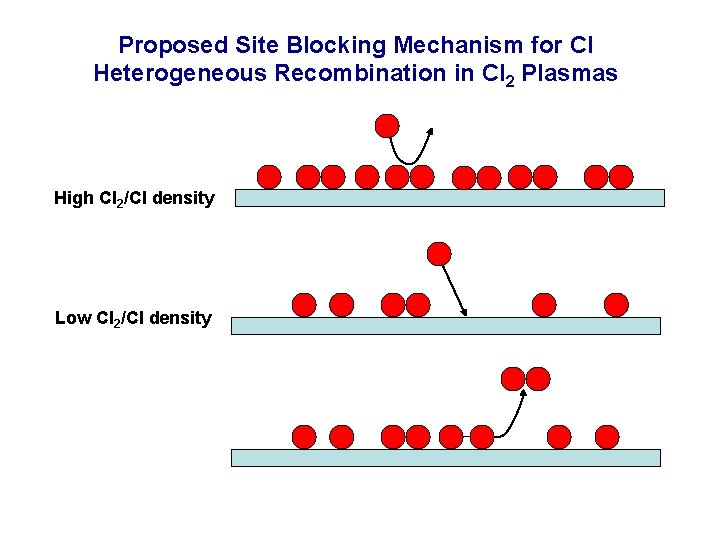
Proposed Site Blocking Mechanism for Cl Heterogeneous Recombination in Cl 2 Plasmas High Cl 2/Cl density Low Cl 2/Cl density

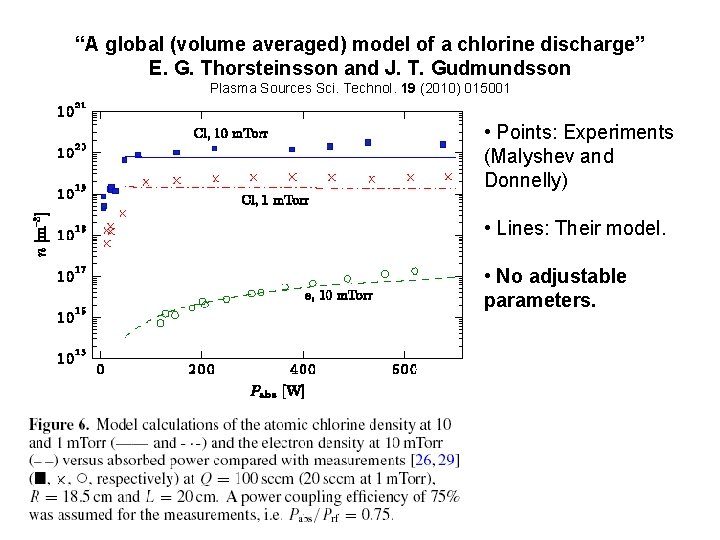
“A global (volume averaged) model of a chlorine discharge” E. G. Thorsteinsson and J. T. Gudmundsson Plasma Sources Sci. Technol. 19 (2010) 015001 • Points: Experiments (Malyshev and Donnelly) • Lines: Their model. • No adjustable parameters.

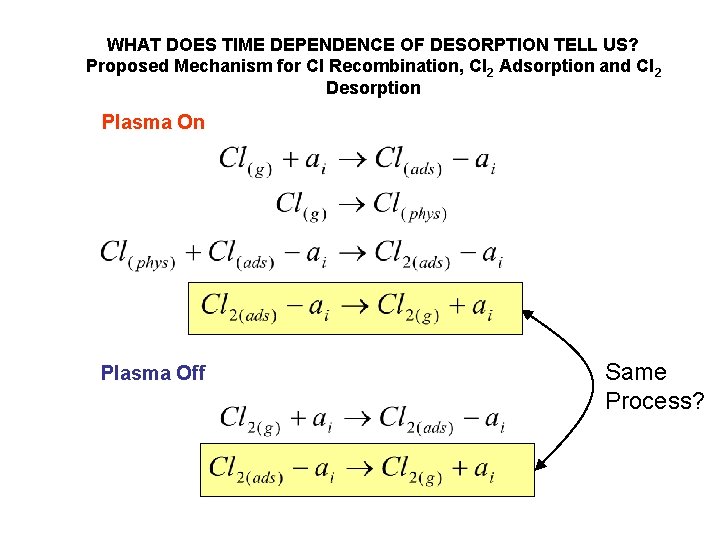
WHAT DOES TIME DEPENDENCE OF DESORPTION TELL US? Proposed Mechanism for Cl Recombination, Cl 2 Adsorption and Cl 2 Desorption Plasma Off Same Process?

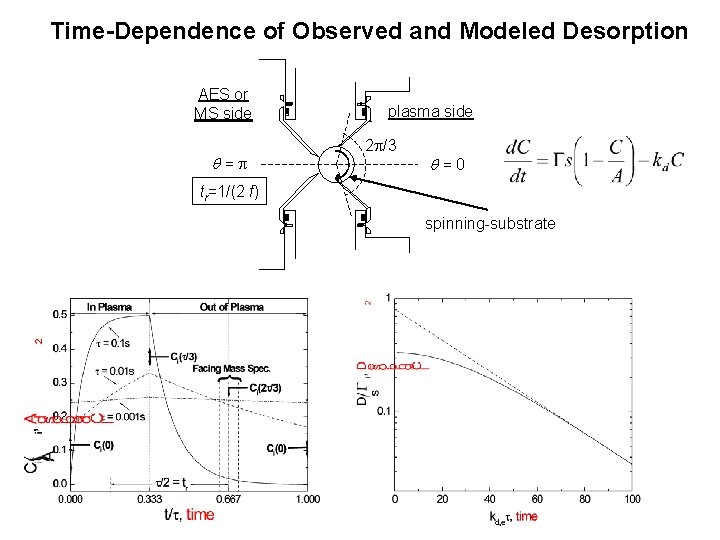
Time-Dependence of Observed and Modeled Desorption AES or MS side = plasma side 2 /3 =0 tr=1/(2 f) spinning-substrate

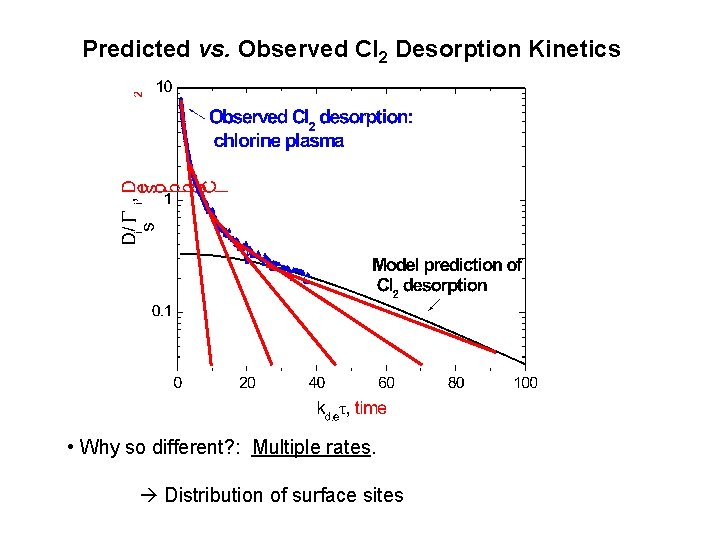
Predicted vs. Observed Cl 2 Desorption Kinetics • Why so different? : Multiple rates. Distribution of surface sites

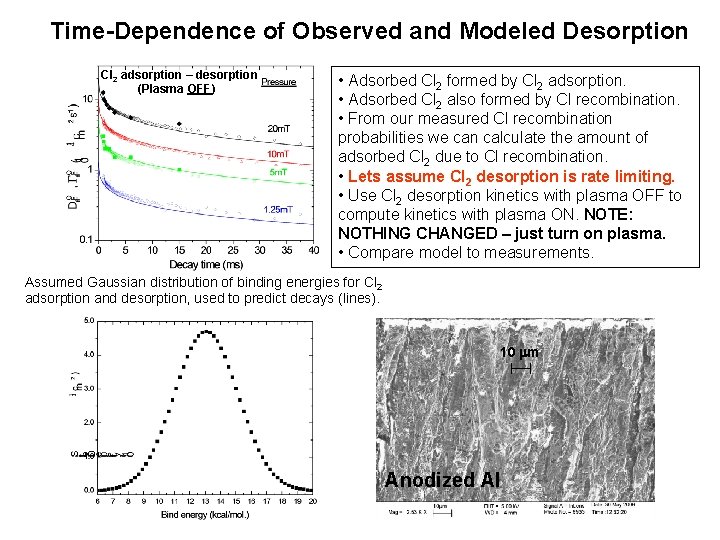
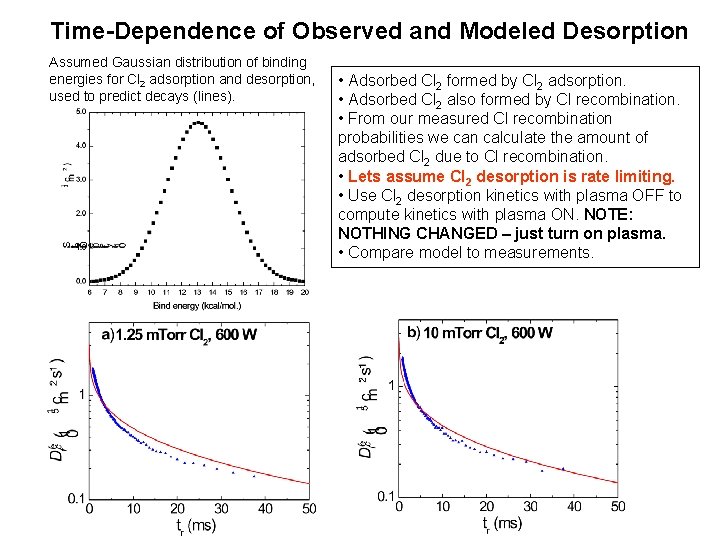
Time-Dependence of Observed and Modeled Desorption Cl 2 adsorption – desorption (Plasma OFF) • Adsorbed Cl 2 formed by Cl 2 adsorption. • Adsorbed Cl 2 also formed by Cl recombination. • From our measured Cl recombination probabilities we can calculate the amount of adsorbed Cl 2 due to Cl recombination. • Lets assume Cl 2 desorption is rate limiting. • Use Cl 2 desorption kinetics with plasma OFF to compute kinetics with plasma ON. NOTE: NOTHING CHANGED – just turn on plasma. • Compare model to measurements. Assumed Gaussian distribution of binding energies for Cl 2 adsorption and desorption, used to predict decays (lines). 10 m Anodized Al

Time-Dependence of Observed and Modeled Desorption Assumed Gaussian distribution of binding energies for Cl 2 adsorption and desorption, used to predict decays (lines). • Adsorbed Cl 2 formed by Cl 2 adsorption. • Adsorbed Cl 2 also formed by Cl recombination. • From our measured Cl recombination probabilities we can calculate the amount of adsorbed Cl 2 due to Cl recombination. • Lets assume Cl 2 desorption is rate limiting. • Use Cl 2 desorption kinetics with plasma OFF to compute kinetics with plasma ON. NOTE: NOTHING CHANGED – just turn on plasma. • Compare model to measurements.

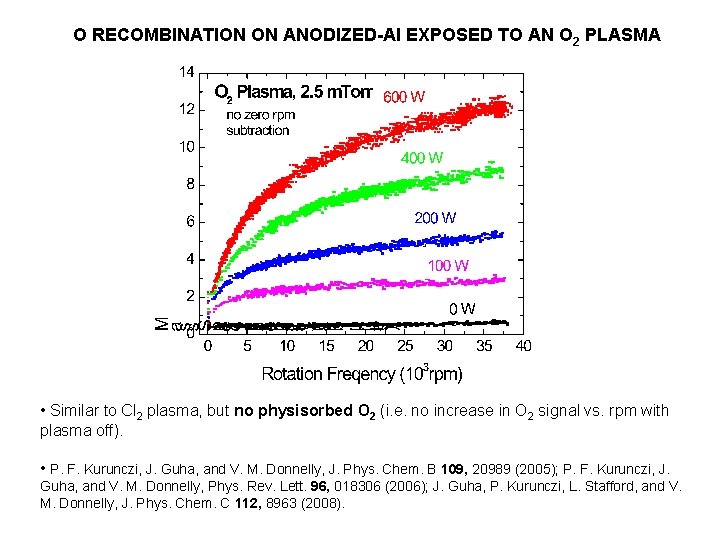
O RECOMBINATION ON ANODIZED-Al EXPOSED TO AN O 2 PLASMA • Similar to Cl 2 plasma, but no physisorbed O 2 (i. e. no increase in O 2 signal vs. rpm with plasma off). • P. F. Kurunczi, J. Guha, and V. M. Donnelly, J. Phys. Chem. B 109, 20989 (2005); P. F. Kurunczi, J. Guha, and V. M. Donnelly, Phys. Rev. Lett. 96, 018306 (2006); J. Guha, P. Kurunczi, L. Stafford, and V. M. Donnelly, J. Phys. Chem. C 112, 8963 (2008).

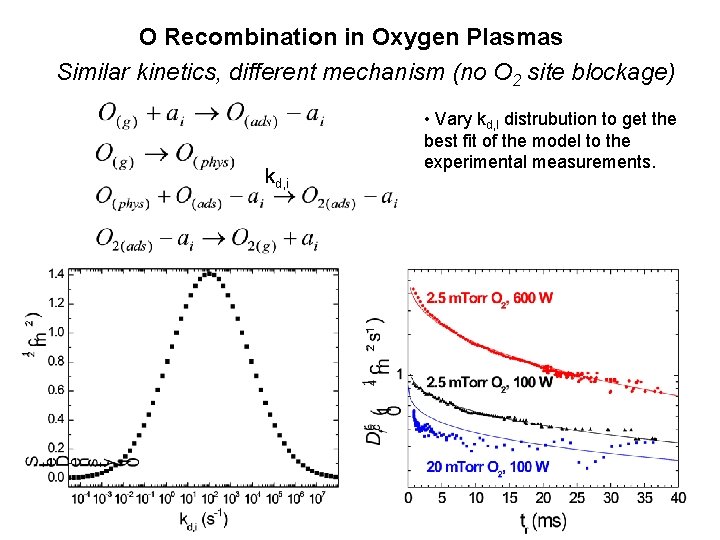
O Recombination in Oxygen Plasmas Similar kinetics, different mechanism (no O 2 site blockage) kd, i • Vary kd, I distrubution to get the best fit of the model to the experimental measurements.

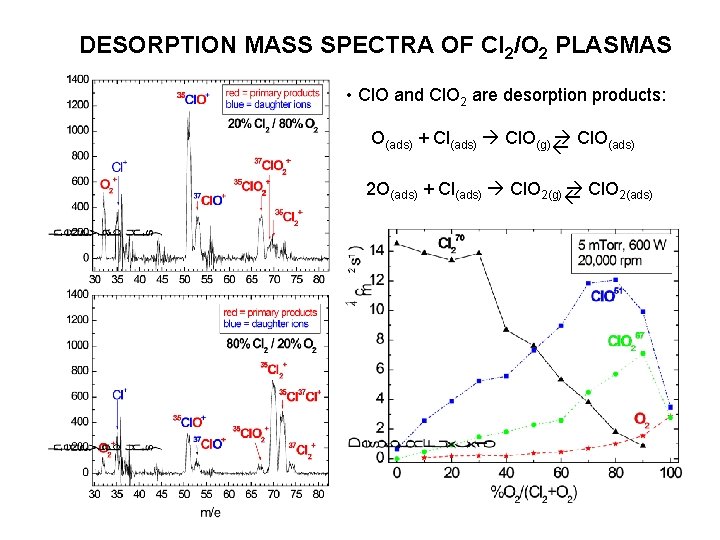
DESORPTION MASS SPECTRA OF Cl 2/O 2 PLASMAS • Cl. O and Cl. O 2 are desorption products: O(ads) + Cl(ads) Cl. O(g) Cl. O(ads) 2 O(ads) + Cl(ads) Cl. O 2(g) Cl. O 2(ads)

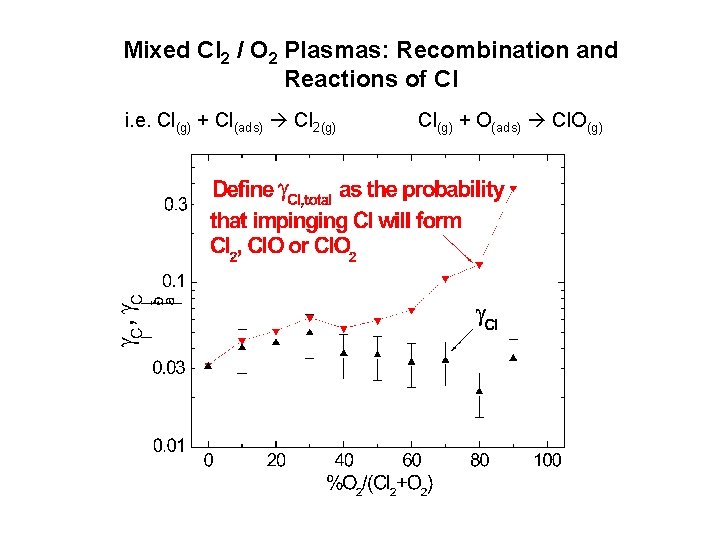
Mixed Cl 2 / O 2 Plasmas: Recombination and Reactions of Cl i. e. Cl(g) + Cl(ads) Cl 2(g) Cl(g) + O(ads) Cl. O(g)

Mixed Cl 2 / O 2 Plasmas: Why does O 2 addition have little effect on Cl, yet addition of Cl 2 suppresses Cl?

Proposed Site Blocking Mechanism for Cl Heterogeneous Recombination in Cl 2 Plasmas Cl 2 sticks and blocks sites for Cl recombination O 2 does not

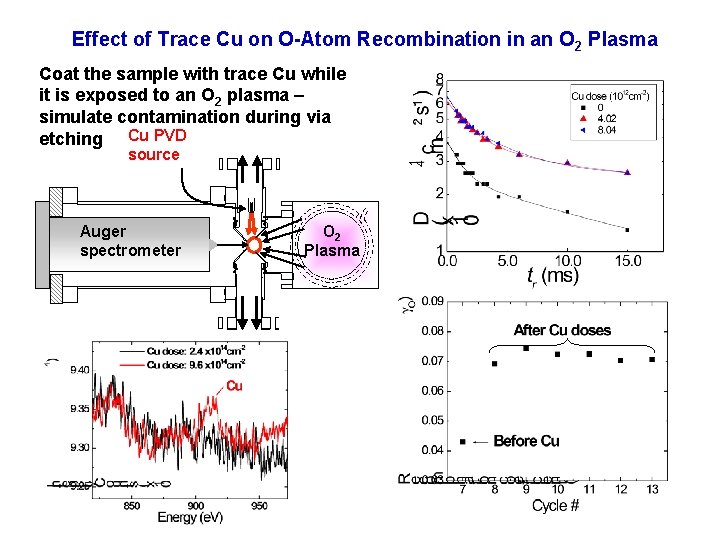
Effect of Trace Cu on O-Atom Recombination in an O 2 Plasma Coat the sample with trace Cu while it is exposed to an O 2 plasma – simulate contamination during via etching Cu PVD source Auger spectrometer O 2 Plasma

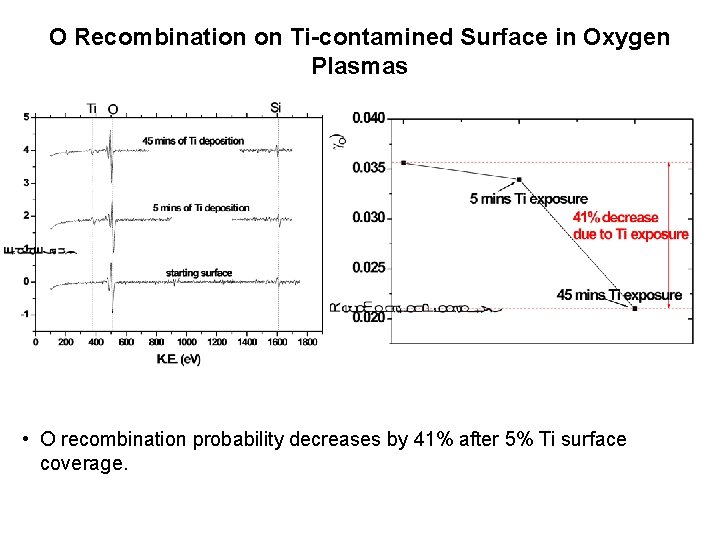
O Recombination on Ti-contamined Surface in Oxygen Plasmas • O recombination probability decreases by 41% after 5% Ti surface coverage.

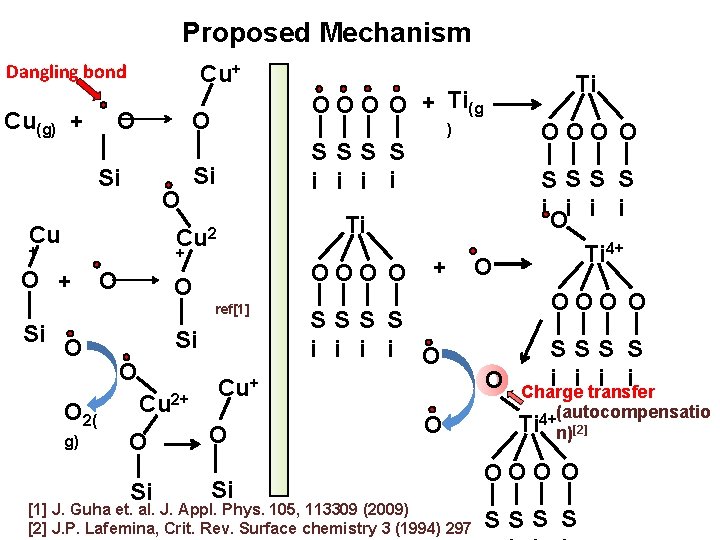
Proposed Mechanism Dangling bond Cu(g) + Cu+ O O Si O Cu + O OOO O O ref[1] Si O O 2( g) Si O Cu 2+ O Si OOO O ) SSS S i Oi i i Ti Cu 2 + O O + Ti(g SSS S i i Si Ti + SSS S i i O Cu+ O Si O [1] J. Guha et. al. J. Appl. Phys. 105, 113309 (2009) [2] J. P. Lafemina, Crit. Rev. Surface chemistry 3 (1994) 297 Ti 4+ O OOO O SSS S i i transfer O Charge (autocompensatio 4+ Ti n)[2] OOO O SSS S

SUMMARY • Cl Langmuir-Hinshelwood (L-H) recombination seems to be limited by Cl 2 desorption. • The mean binding energy for Cl 2 on anodized Al is 13 kcal/mol. and the range of binding energies is ~9 to 17 kcal/mol. • Cl recombination coefficient increases with Cl-to-Cl 2 number density ratio. • O recombination on anodized Al follows kinetics with a range of rates at distributions of sites, but the mechanism is different from Cl recombination – no O 2 site blockage. • Our values have been used in a global model by Thorsteinsson and Gudmundsson. With no adjustable parameters, their model reproduces Cl densities measure by Maylshev and Donnelly in a chlorine ICP. • Trace Cu surface contamination catalyzes O recombination. • Small amounts of surface Ti suppresses O recombination.