NEW OPPORTUNITIES IN PLASMASURFACE INTERACTIONS FOR FUNCTIONALIZATION OF



![SURFACE INTERACTIONS: [O] DENSITY 1 x 109 - 1 x 1012 5 x 1010 SURFACE INTERACTIONS: [O] DENSITY 1 x 109 - 1 x 1012 5 x 1010](https://slidetodoc.com/presentation_image_h/9e3d6d3858d5806dcf6f7ced011e759c/image-37.jpg)
![COVERAGE OF PEROXY [=C-O-O ] BY BONDING AT 10 ms · Primary and secondary COVERAGE OF PEROXY [=C-O-O ] BY BONDING AT 10 ms · Primary and secondary](https://slidetodoc.com/presentation_image_h/9e3d6d3858d5806dcf6f7ced011e759c/image-43.jpg)
![COVERAGE OF PEROXY [=C-O-O ] BY BONDING AT 140 ms · Long term production COVERAGE OF PEROXY [=C-O-O ] BY BONDING AT 140 ms · Long term production](https://slidetodoc.com/presentation_image_h/9e3d6d3858d5806dcf6f7ced011e759c/image-44.jpg)
- Slides: 50

NEW OPPORTUNITIES IN PLASMA-SURFACE INTERACTIONS FOR FUNCTIONALIZATION OF SURFACES* Ananth Bhoj, Natalie Babaeva, Rajesh Dorai and Mark J. Kushner Iowa State University 104 Marston Hall Ames, IA 50011 mjk@iastate. edu http: //uigelz. ece. iastate. edu May 2005 *Work supported by National Science Foundation, 3 M Inc. DAMOP_0505_01

AGENDA · Plasmas for modification of surfaces · Functionalization of polymers · Challenges for adapting commodity processes for high value materials. · Opportunities for AMO · Concluding Remarks DAMOP_0505_02 Iowa State University Optical and Discharge Physics

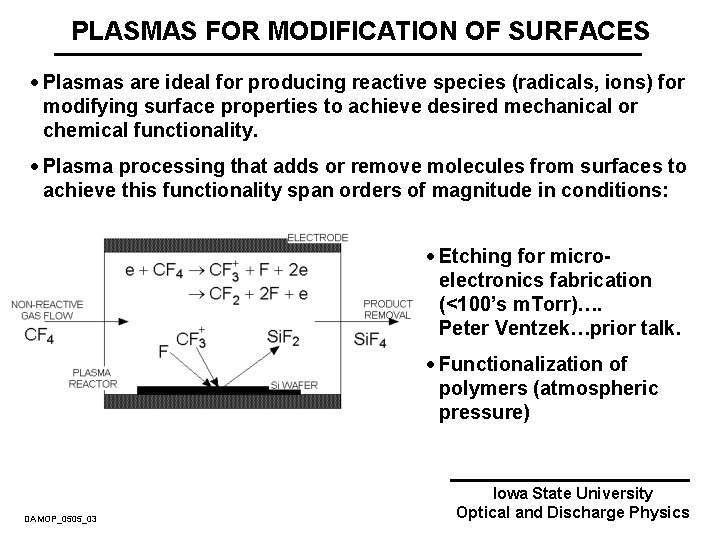
PLASMAS FOR MODIFICATION OF SURFACES · Plasmas are ideal for producing reactive species (radicals, ions) for modifying surface properties to achieve desired mechanical or chemical functionality. · Plasma processing that adds or remove molecules from surfaces to achieve this functionality span orders of magnitude in conditions: · Etching for microelectronics fabrication (<100’s m. Torr)…. Peter Ventzek…prior talk. . DAMOP_0505_03 · Functionalization of polymers (atmospheric pressure) Iowa State University Optical and Discharge Physics

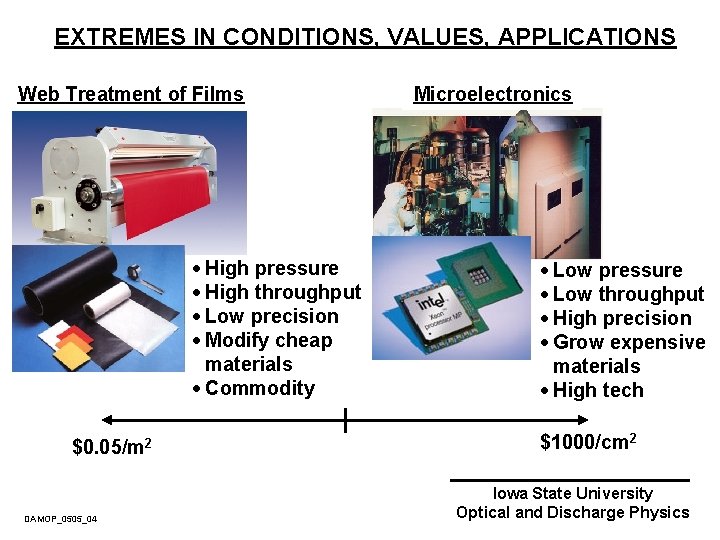
EXTREMES IN CONDITIONS, VALUES, APPLICATIONS Web Treatment of Films · High pressure · High throughput · Low precision · Modify cheap materials · Commodity $0. 05/m 2 DAMOP_0505_04 Microelectronics · Low pressure · Low throughput · High precision · Grow expensive materials · High tech $1000/cm 2 Iowa State University Optical and Discharge Physics

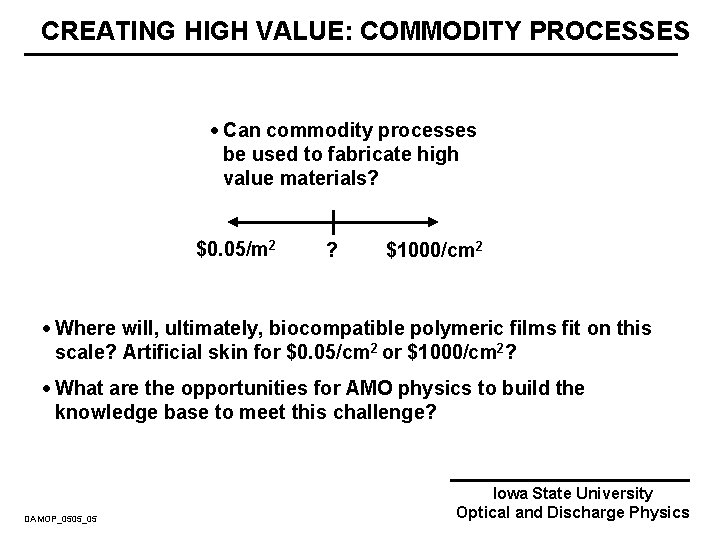
CREATING HIGH VALUE: COMMODITY PROCESSES · Can commodity processes be used to fabricate high value materials? $0. 05/m 2 ? $1000/cm 2 · Where will, ultimately, biocompatible polymeric films fit on this scale? Artificial skin for $0. 05/cm 2 or $1000/cm 2? · What are the opportunities for AMO physics to build the knowledge base to meet this challenge? DAMOP_0505_05 Iowa State University Optical and Discharge Physics

LOW COST, COMMODITY FUNCTIONALIZATION OF POLYMERS DAMOP_0505_06

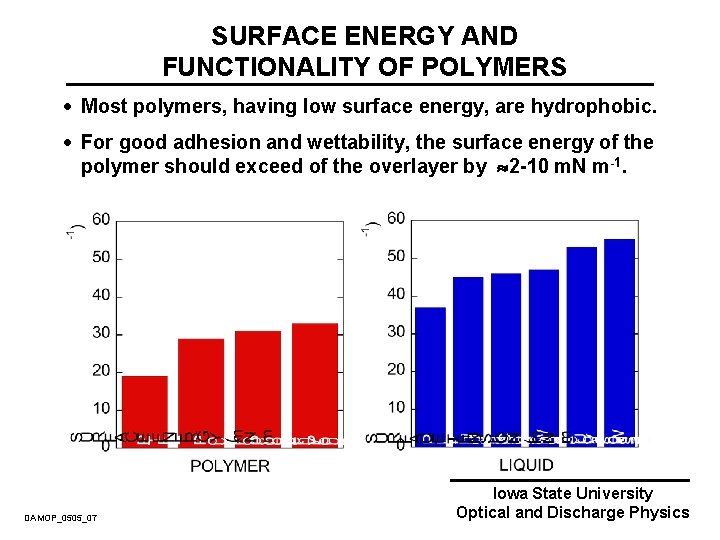
SURFACE ENERGY AND FUNCTIONALITY OF POLYMERS · Most polymers, having low surface energy, are hydrophobic. · For good adhesion and wettability, the surface energy of the polymer should exceed of the overlayer by 2 -10 m. N m-1. DAMOP_0505_07 Iowa State University Optical and Discharge Physics

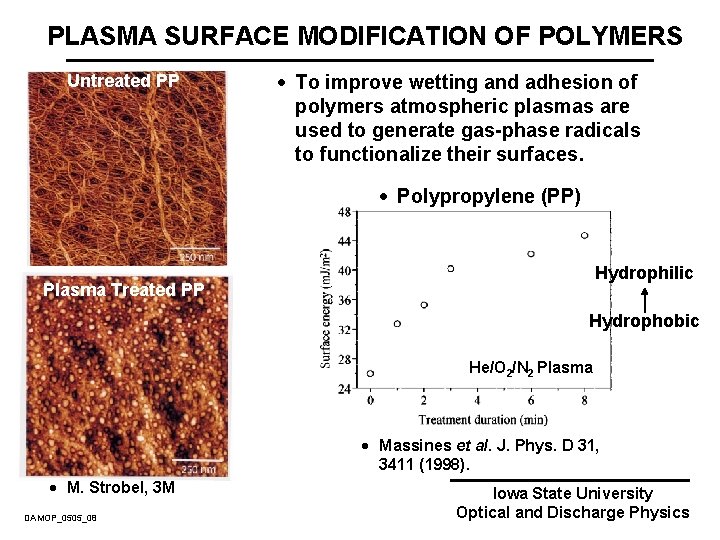
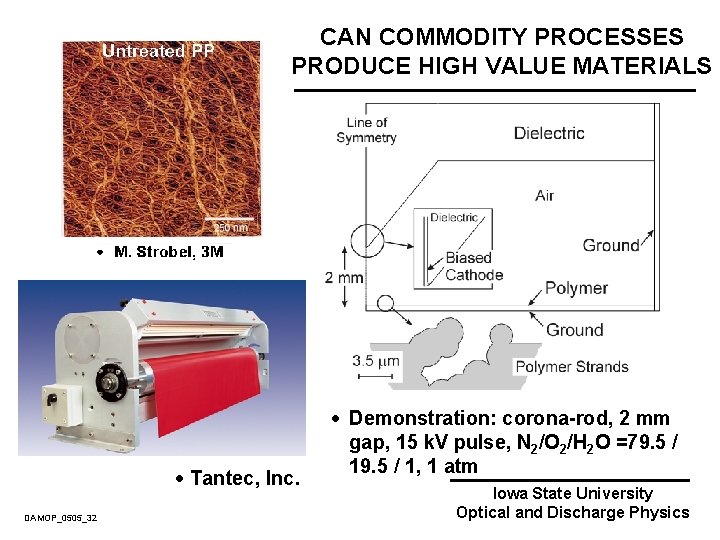
PLASMA SURFACE MODIFICATION OF POLYMERS Untreated PP · To improve wetting and adhesion of polymers atmospheric plasmas are used to generate gas-phase radicals to functionalize their surfaces. · Polypropylene (PP) Hydrophilic Plasma Treated PP Hydrophobic He/O 2/N 2 Plasma · Massines et al. J. Phys. D 31, 3411 (1998). · M. Strobel, 3 M DAMOP_0505_08 Iowa State University Optical and Discharge Physics

POLYMER TREATMENT APPARATUS · Filamentary Plasma 10 s – 200 m DAMOP_0505_09 Iowa State University Optical and Discharge Physics

COMMERCIAL CORONA PLASMA EQUIPMENT · Sherman Treaters · Tantec, Inc. DAMOP_0505_10 Iowa State University Optical and Discharge Physics

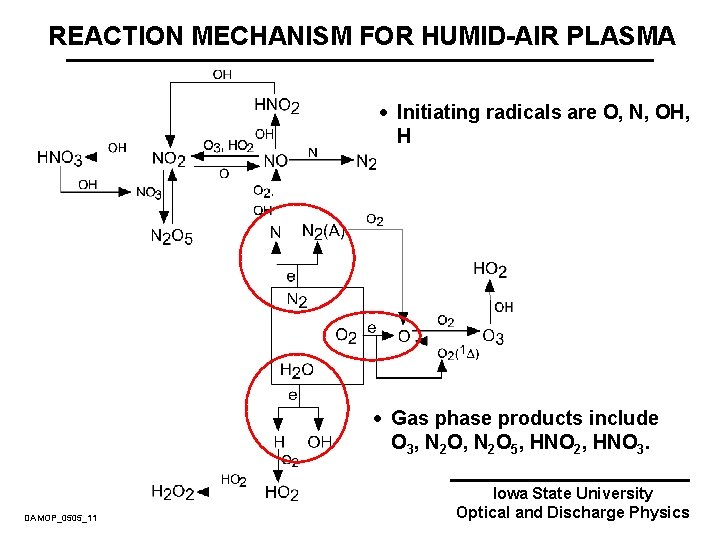
REACTION MECHANISM FOR HUMID-AIR PLASMA · Initiating radicals are O, N, OH, H · Gas phase products include O 3, N 2 O 5, HNO 2, HNO 3. DAMOP_0505_11 Iowa State University Optical and Discharge Physics

REACTION PATHWAY DAMOP_0505_12 Iowa State University Optical and Discharge Physics

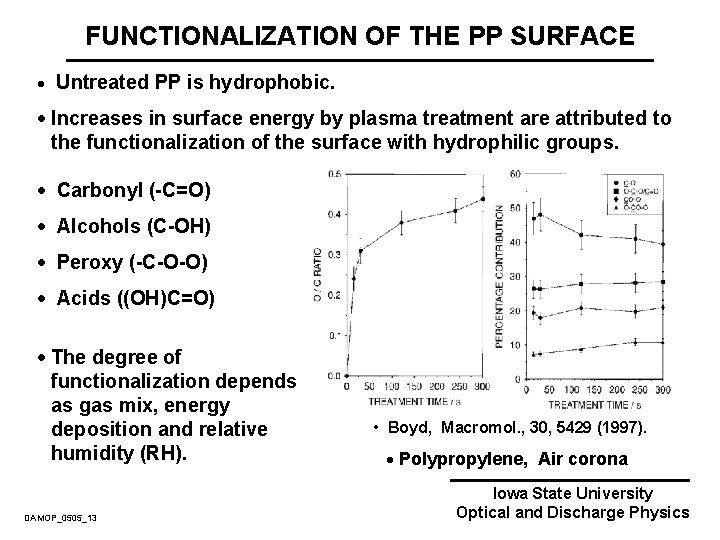
FUNCTIONALIZATION OF THE PP SURFACE · Untreated PP is hydrophobic. · Increases in surface energy by plasma treatment are attributed to the functionalization of the surface with hydrophilic groups. · Carbonyl (-C=O) · Alcohols (C-OH) · Peroxy (-C-O-O) · Acids ((OH)C=O) · The degree of functionalization depends as gas mix, energy deposition and relative humidity (RH). DAMOP_0505_13 • Boyd, Macromol. , 30, 5429 (1997). · Polypropylene, Air corona Iowa State University Optical and Discharge Physics

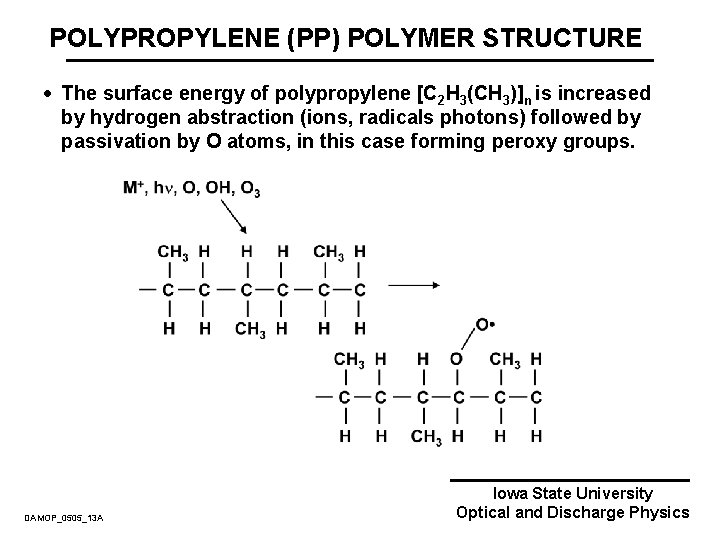
POLYPROPYLENE (PP) POLYMER STRUCTURE · The surface energy of polypropylene [C 2 H 3(CH 3)]n is increased by hydrogen abstraction (ions, radicals photons) followed by passivation by O atoms, in this case forming peroxy groups. DAMOP_0505_13 A Iowa State University Optical and Discharge Physics

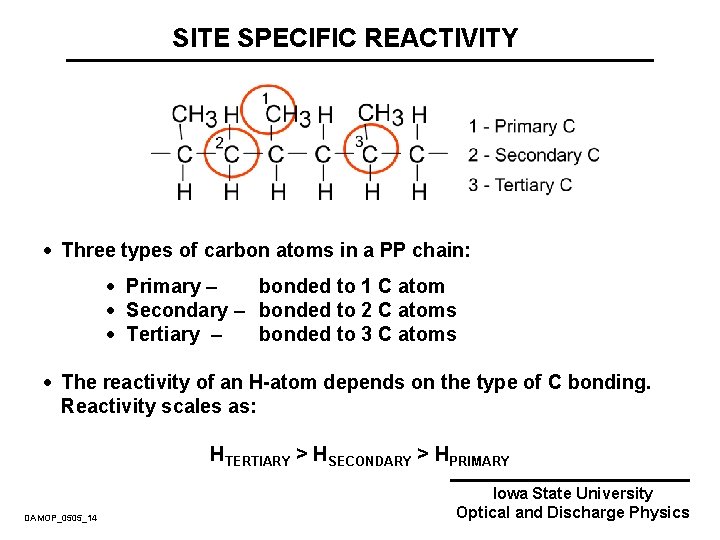
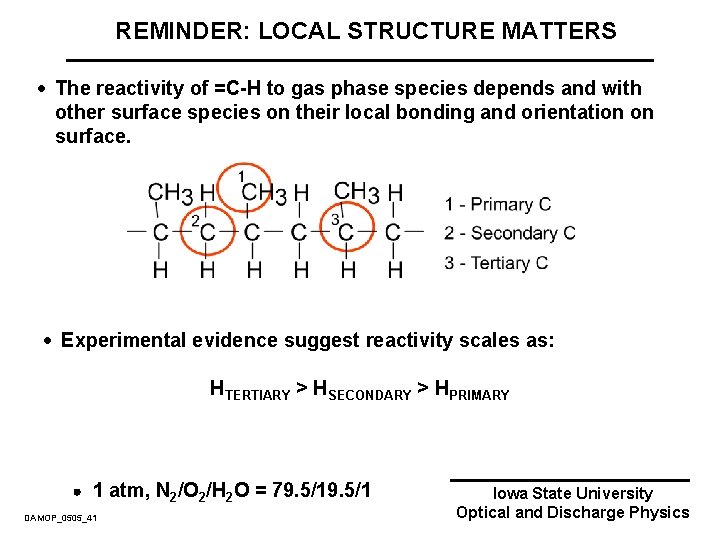
SITE SPECIFIC REACTIVITY · Three types of carbon atoms in a PP chain: · Primary – bonded to 1 C atom · Secondary – bonded to 2 C atoms · Tertiary – bonded to 3 C atoms · The reactivity of an H-atom depends on the type of C bonding. Reactivity scales as: HTERTIARY > HSECONDARY > HPRIMARY DAMOP_0505_14 Iowa State University Optical and Discharge Physics

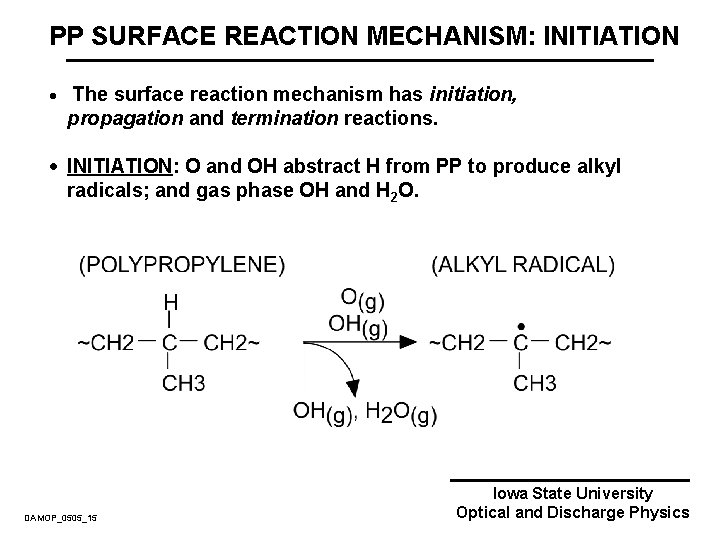
PP SURFACE REACTION MECHANISM: INITIATION · The surface reaction mechanism has initiation, propagation and termination reactions. · INITIATION: O and OH abstract H from PP to produce alkyl radicals; and gas phase OH and H 2 O. DAMOP_0505_15 Iowa State University Optical and Discharge Physics

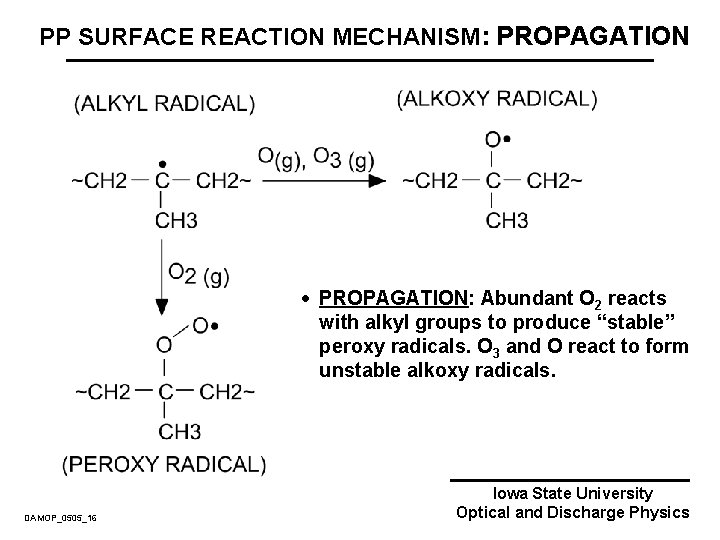
PP SURFACE REACTION MECHANISM: PROPAGATION · PROPAGATION: Abundant O 2 reacts with alkyl groups to produce “stable” peroxy radicals. O 3 and O react to form unstable alkoxy radicals. DAMOP_0505_16 Iowa State University Optical and Discharge Physics

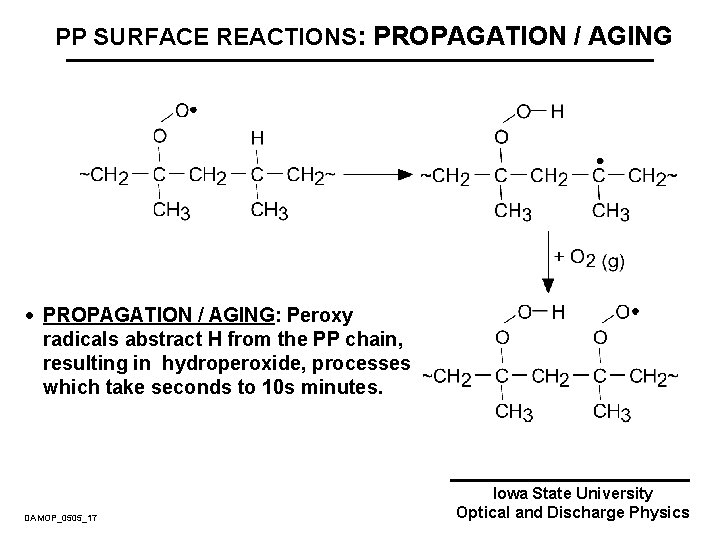
PP SURFACE REACTIONS: PROPAGATION / AGING · PROPAGATION / AGING: Peroxy radicals abstract H from the PP chain, resulting in hydroperoxide, processes which take seconds to 10 s minutes. DAMOP_0505_17 Iowa State University Optical and Discharge Physics

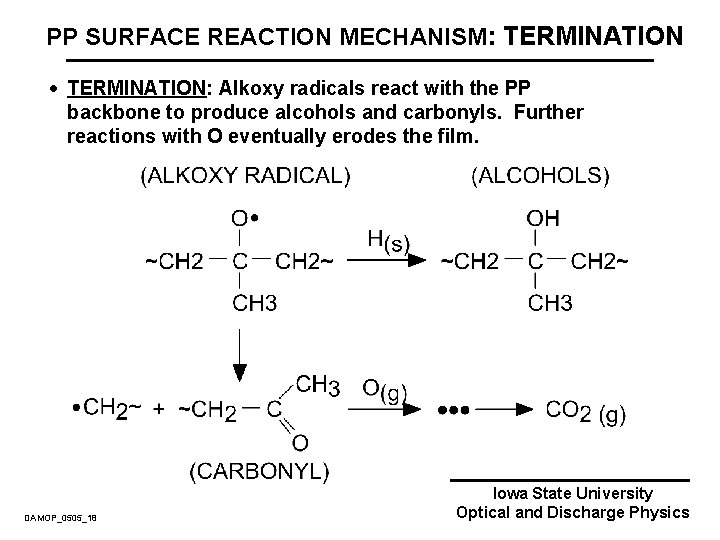
PP SURFACE REACTION MECHANISM: TERMINATION · TERMINATION: Alkoxy radicals react with the PP backbone to produce alcohols and carbonyls. Further reactions with O eventually erodes the film. DAMOP_0505_18 Iowa State University Optical and Discharge Physics

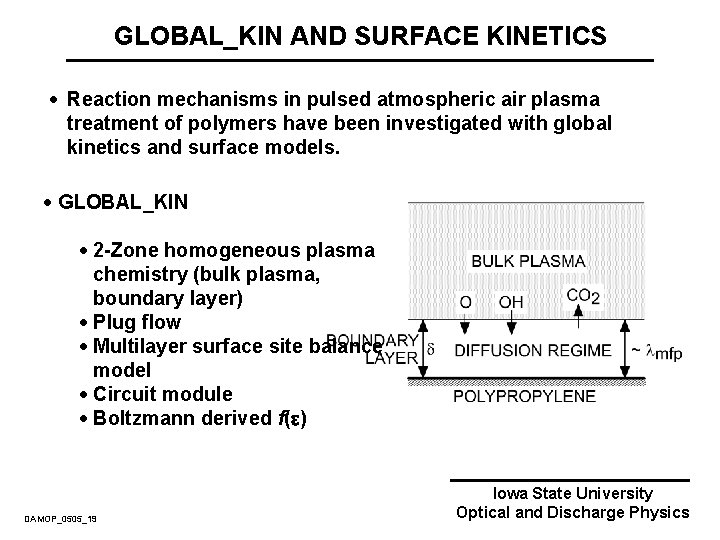
GLOBAL_KIN AND SURFACE KINETICS · Reaction mechanisms in pulsed atmospheric air plasma treatment of polymers have been investigated with global kinetics and surface models. · GLOBAL_KIN · 2 -Zone homogeneous plasma chemistry (bulk plasma, boundary layer) · Plug flow · Multilayer surface site balance model · Circuit module · Boltzmann derived f( ) DAMOP_0505_19 Iowa State University Optical and Discharge Physics

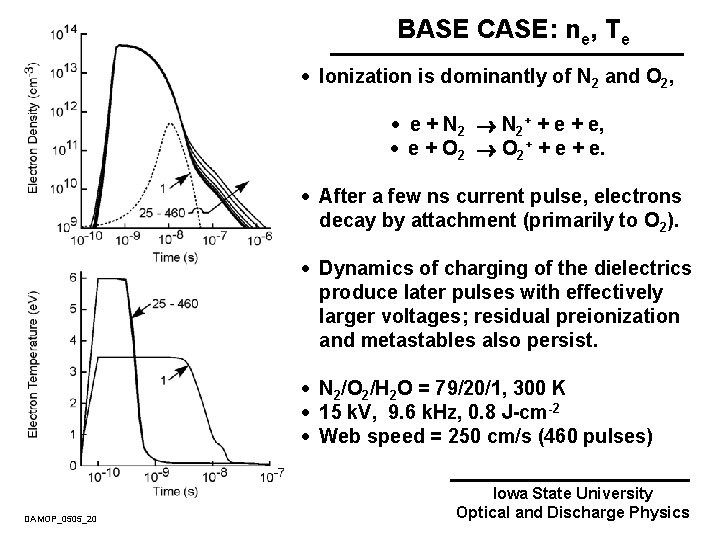
BASE CASE: ne, Te · Ionization is dominantly of N 2 and O 2, · e + N 2+ + e, · e + O 2+ + e. · After a few ns current pulse, electrons decay by attachment (primarily to O 2). · Dynamics of charging of the dielectrics produce later pulses with effectively larger voltages; residual preionization and metastables also persist. · N 2/O 2/H 2 O = 79/20/1, 300 K · 15 k. V, 9. 6 k. Hz, 0. 8 J-cm-2 · Web speed = 250 cm/s (460 pulses) DAMOP_0505_20 Iowa State University Optical and Discharge Physics

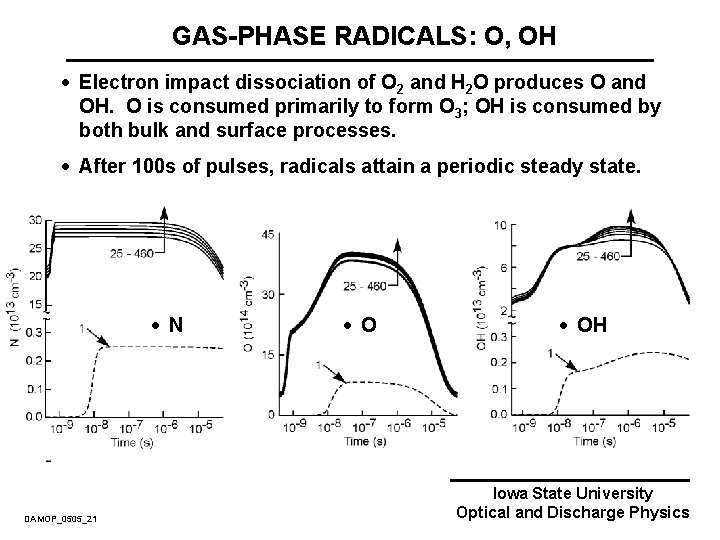
GAS-PHASE RADICALS: O, OH · Electron impact dissociation of O 2 and H 2 O produces O and OH. O is consumed primarily to form O 3; OH is consumed by both bulk and surface processes. · After 100 s of pulses, radicals attain a periodic steady state. · N DAMOP_0505_21 · OH Iowa State University Optical and Discharge Physics

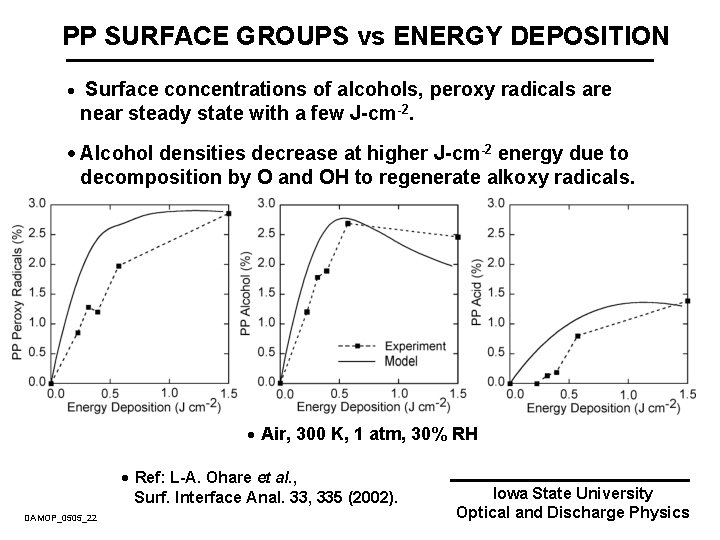
PP SURFACE GROUPS vs ENERGY DEPOSITION · Surface concentrations of alcohols, peroxy radicals are near steady state with a few J-cm-2. · Alcohol densities decrease at higher J-cm-2 energy due to decomposition by O and OH to regenerate alkoxy radicals. · Air, 300 K, 1 atm, 30% RH · Ref: L-A. Ohare et al. , Surf. Interface Anal. 33, 335 (2002). DAMOP_0505_22 Iowa State University Optical and Discharge Physics

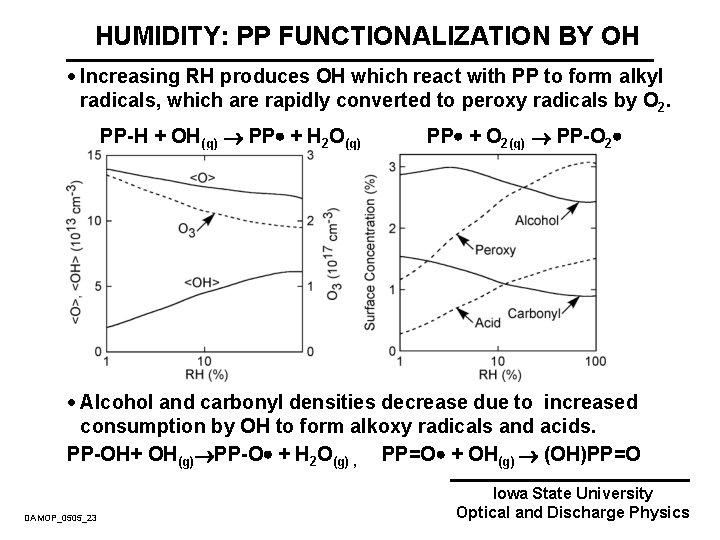
HUMIDITY: PP FUNCTIONALIZATION BY OH · Increasing RH produces OH which react with PP to form alkyl radicals, which are rapidly converted to peroxy radicals by O 2. PP-H + OH(g) PP + H 2 O(g) PP + O 2(g) PP-O 2 · Alcohol and carbonyl densities decrease due to increased consumption by OH to form alkoxy radicals and acids. PP-OH+ OH(g) PP-O + H 2 O(g) , PP=O + OH(g) (OH)PP=O DAMOP_0505_23 Iowa State University Optical and Discharge Physics

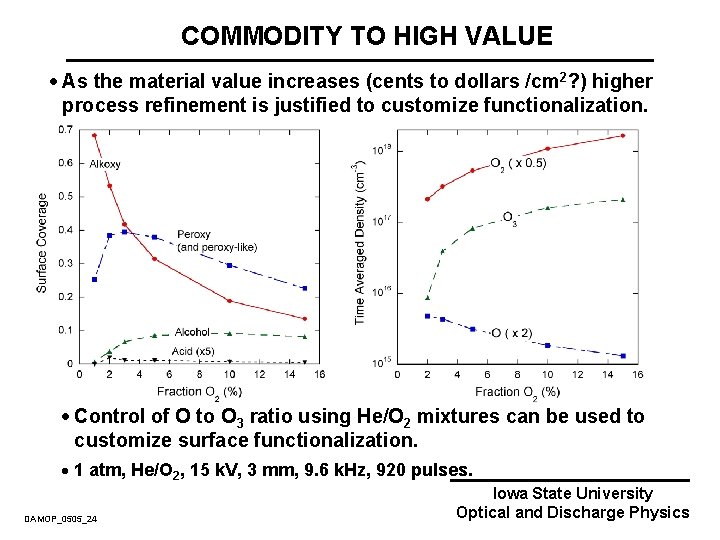
COMMODITY TO HIGH VALUE · As the material value increases (cents to dollars /cm 2? ) higher process refinement is justified to customize functionalization. · Control of O to O 3 ratio using He/O 2 mixtures can be used to customize surface functionalization. · 1 atm, He/O 2, 15 k. V, 3 mm, 9. 6 k. Hz, 920 pulses. DAMOP_0505_24 Iowa State University Optical and Discharge Physics

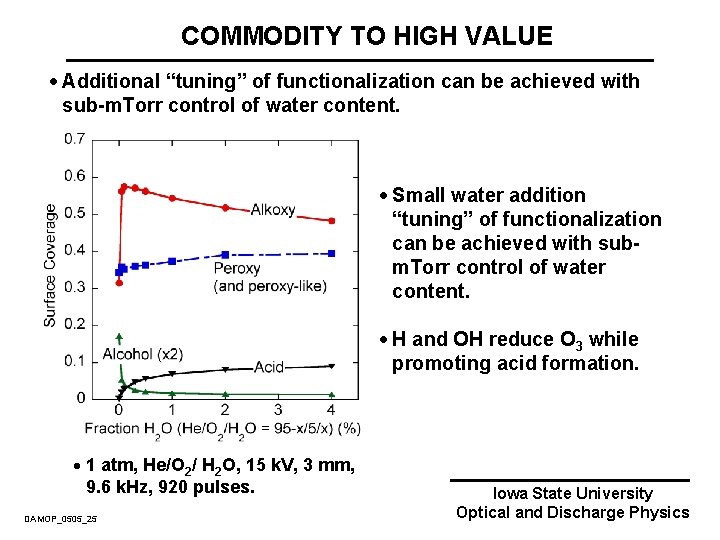
COMMODITY TO HIGH VALUE · Additional “tuning” of functionalization can be achieved with sub-m. Torr control of water content. · Small water addition “tuning” of functionalization can be achieved with subm. Torr control of water content. · H and OH reduce O 3 while promoting acid formation. · 1 atm, He/O 2/ H 2 O, 15 k. V, 3 mm, 9. 6 k. Hz, 920 pulses. DAMOP_0505_25 Iowa State University Optical and Discharge Physics

THE CHALLENGE: COMMODITY PROCESSING FOR HIGH VALUE MATERIALS DAMOP_0505_26

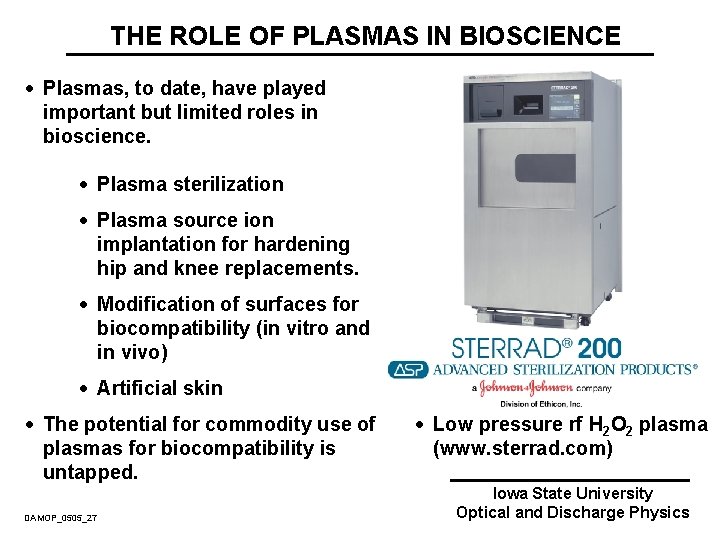
THE ROLE OF PLASMAS IN BIOSCIENCE · Plasmas, to date, have played important but limited roles in bioscience. · Plasma sterilization · Plasma source ion implantation for hardening hip and knee replacements. · Modification of surfaces for biocompatibility (in vitro and in vivo) · Artificial skin · The potential for commodity use of plasmas for biocompatibility is untapped. DAMOP_0505_27 · Low pressure rf H 2 O 2 plasma (www. sterrad. com) Iowa State University Optical and Discharge Physics

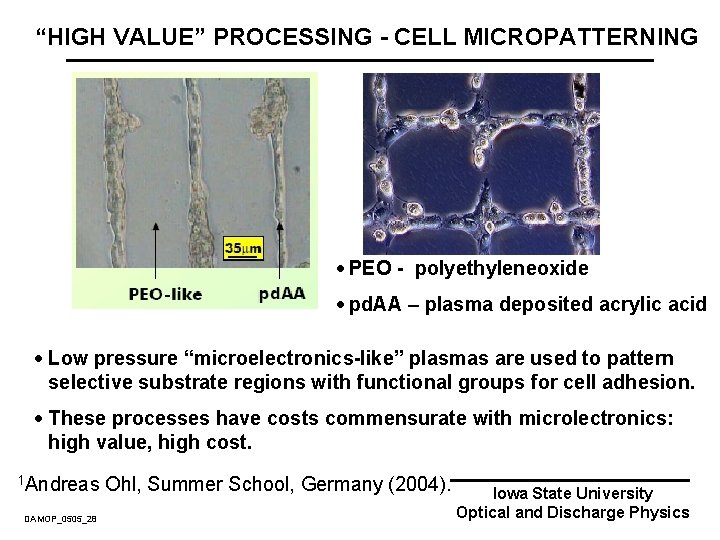
“HIGH VALUE” PROCESSING - CELL MICROPATTERNING · PEO - polyethyleneoxide · pd. AA – plasma deposited acrylic acid · Low pressure “microelectronics-like” plasmas are used to pattern selective substrate regions with functional groups for cell adhesion. · These processes have costs commensurate with microlectronics: high value, high cost. 1 Andreas DAMOP_0505_28 Ohl, Summer School, Germany (2004). Iowa State University Optical and Discharge Physics

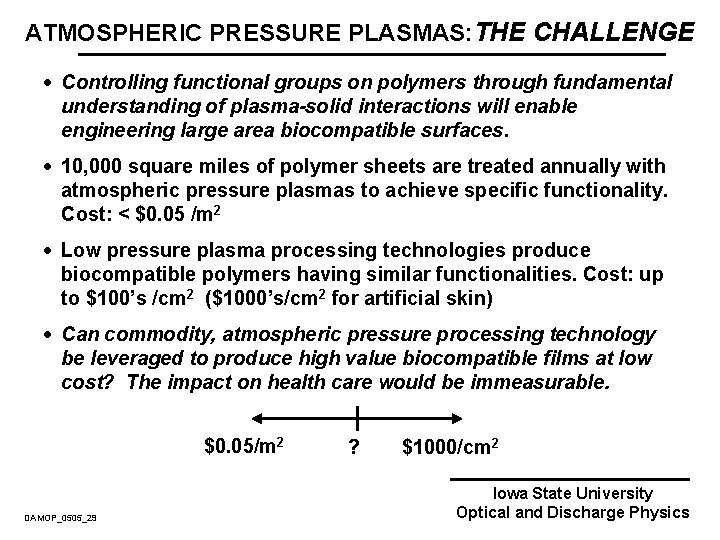
ATMOSPHERIC PRESSURE PLASMAS: THE CHALLENGE · Controlling functional groups on polymers through fundamental understanding of plasma-solid interactions will enable engineering large area biocompatible surfaces. · 10, 000 square miles of polymer sheets are treated annually with atmospheric pressure plasmas to achieve specific functionality. Cost: < $0. 05 /m 2 · Low pressure plasma processing technologies produce biocompatible polymers having similar functionalities. Cost: up to $100’s /cm 2 ($1000’s/cm 2 for artificial skin) · Can commodity, atmospheric pressure processing technology be leveraged to produce high value biocompatible films at low cost? The impact on health care would be immeasurable. $0. 05/m 2 DAMOP_0505_29 ? $1000/cm 2 Iowa State University Optical and Discharge Physics

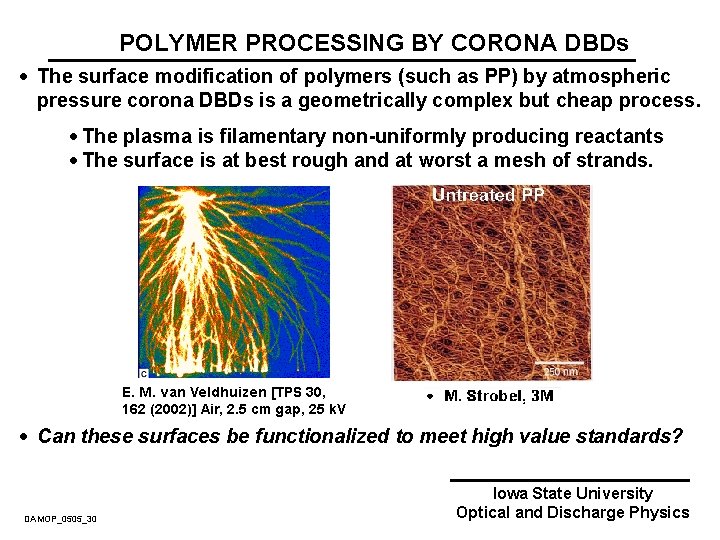
POLYMER PROCESSING BY CORONA DBDs · The surface modification of polymers (such as PP) by atmospheric pressure corona DBDs is a geometrically complex but cheap process. · The plasma is filamentary non-uniformly producing reactants · The surface is at best rough and at worst a mesh of strands. · Can these surfaces be functionalized to meet high value standards? DAMOP_0505_30 Iowa State University Optical and Discharge Physics

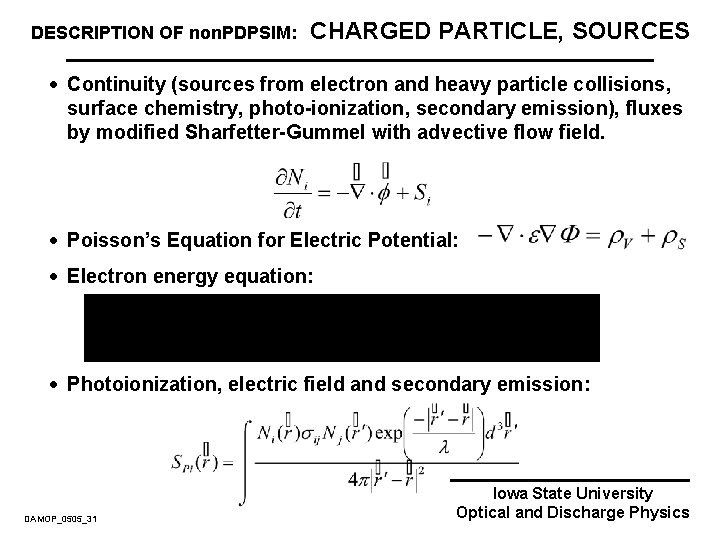
DESCRIPTION OF non. PDPSIM: CHARGED PARTICLE, SOURCES · Continuity (sources from electron and heavy particle collisions, surface chemistry, photo-ionization, secondary emission), fluxes by modified Sharfetter-Gummel with advective flow field. · Poisson’s Equation for Electric Potential: · Electron energy equation: · Photoionization, electric field and secondary emission: DAMOP_0505_31 Iowa State University Optical and Discharge Physics

CAN COMMODITY PROCESSES PRODUCE HIGH VALUE MATERIALS · Tantec, Inc. DAMOP_0505_32 · Demonstration: corona-rod, 2 mm gap, 15 k. V pulse, N 2/O 2/H 2 O =79. 5 / 1, 1 atm Iowa State University Optical and Discharge Physics

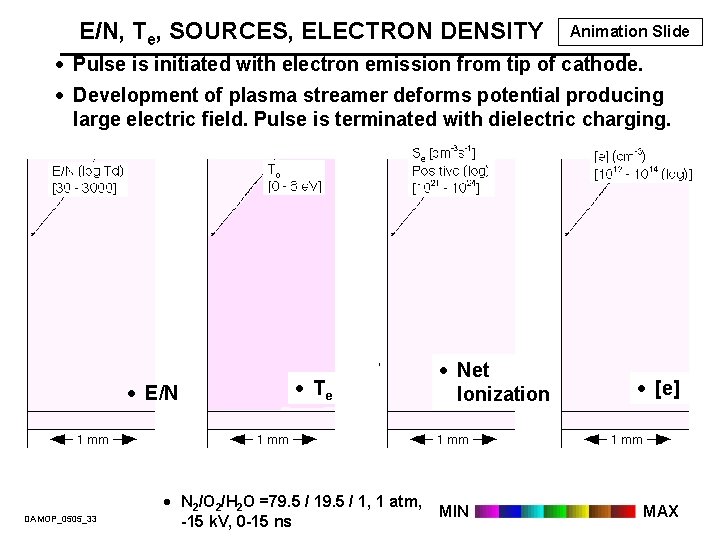
E/N, Te, SOURCES, ELECTRON DENSITY Animation Slide · Pulse is initiated with electron emission from tip of cathode. · Development of plasma streamer deforms potential producing large electric field. Pulse is terminated with dielectric charging. · E/N DAMOP_0505_33 · Te · N 2/O 2/H 2 O =79. 5 / 1, 1 atm, -15 k. V, 0 -15 ns · Net Ionization MIN · [e] MAX

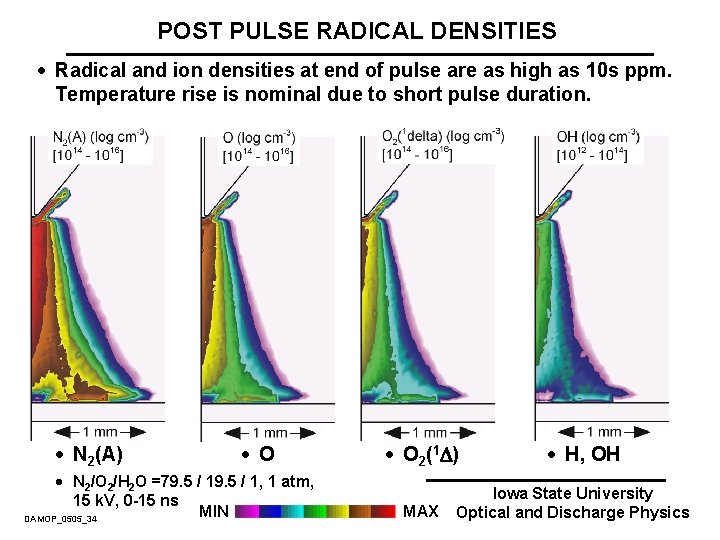
POST PULSE RADICAL DENSITIES · Radical and ion densities at end of pulse are as high as 10 s ppm. Temperature rise is nominal due to short pulse duration. · N 2(A) · O · N 2/O 2/H 2 O =79. 5 / 1, 1 atm, 15 k. V, 0 -15 ns MIN DAMOP_0505_34 · O 2(1 ) MAX · H, OH Iowa State University Optical and Discharge Physics

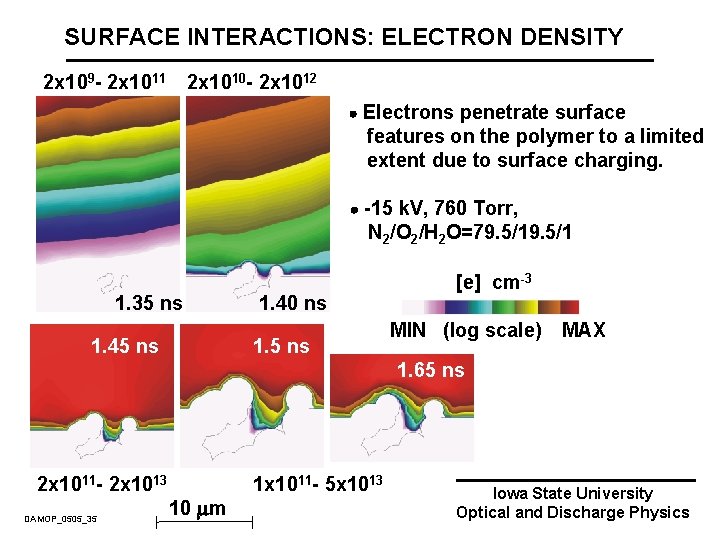
SURFACE INTERACTIONS: ELECTRON DENSITY 2 x 109 - 2 x 1011 2 x 1010 - 2 x 1012 Electrons penetrate surface features on the polymer to a limited extent due to surface charging. -15 k. V, 760 Torr, N 2/O 2/H 2 O=79. 5/1 1. 35 ns 1. 40 ns 1. 5 ns [e] cm-3 MIN (log scale) MAX 1. 65 ns 2 x 1011 - 2 x 1013 DAMOP_0505_35 10 m 1 x 1011 - 5 x 1013 Iowa State University Optical and Discharge Physics
![SURFACE INTERACTIONS O DENSITY 1 x 109 1 x 1012 5 x 1010 SURFACE INTERACTIONS: [O] DENSITY 1 x 109 - 1 x 1012 5 x 1010](https://slidetodoc.com/presentation_image_h/9e3d6d3858d5806dcf6f7ced011e759c/image-37.jpg)
SURFACE INTERACTIONS: [O] DENSITY 1 x 109 - 1 x 1012 5 x 1010 - 5 x 1013 Radicals striking the surface penetrate into the features by diffusion. Unlike charged species, with time, the density of radicals such as [O], increases inside these features. 1. 4 ns 1. 65 ns -15 k. V, 760 Torr, 1. 5 ns 4. 0 ns N 2/O 2/H 2 O=79. 5/1 7. 0 ns [O] cm-3 10 m DAMOP_0505_36 1 x 1011 - 1 x 1014 MIN (log scale) MAX Iowa State University Optical and Discharge Physics

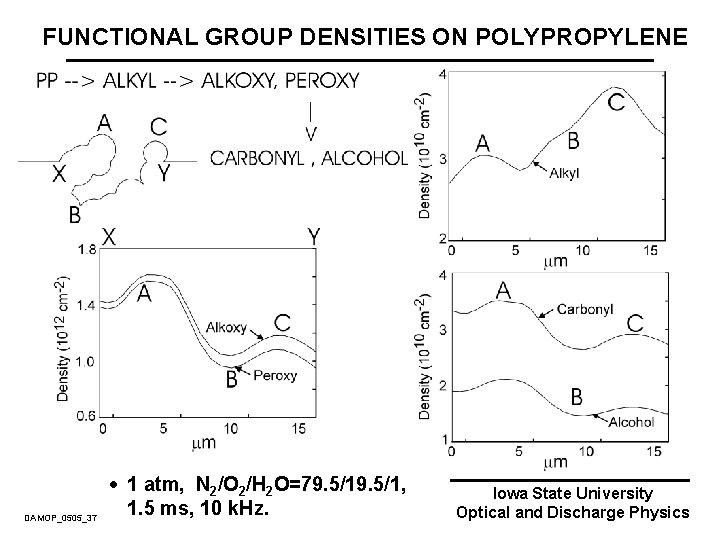
FUNCTIONAL GROUP DENSITIES ON POLYPROPYLENE DAMOP_0505_37 · 1 atm, N 2/O 2/H 2 O=79. 5/1, 1. 5 ms, 10 k. Hz. Iowa State University Optical and Discharge Physics

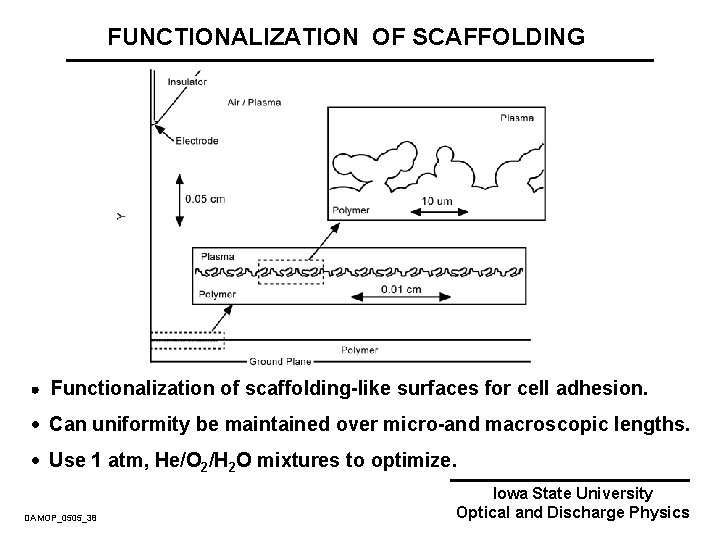
FUNCTIONALIZATION OF SCAFFOLDING Functionalization of scaffolding-like surfaces for cell adhesion. · Can uniformity be maintained over micro-and macroscopic lengths. · Use 1 atm, He/O 2/H 2 O mixtures to optimize. DAMOP_0505_38 Iowa State University Optical and Discharge Physics

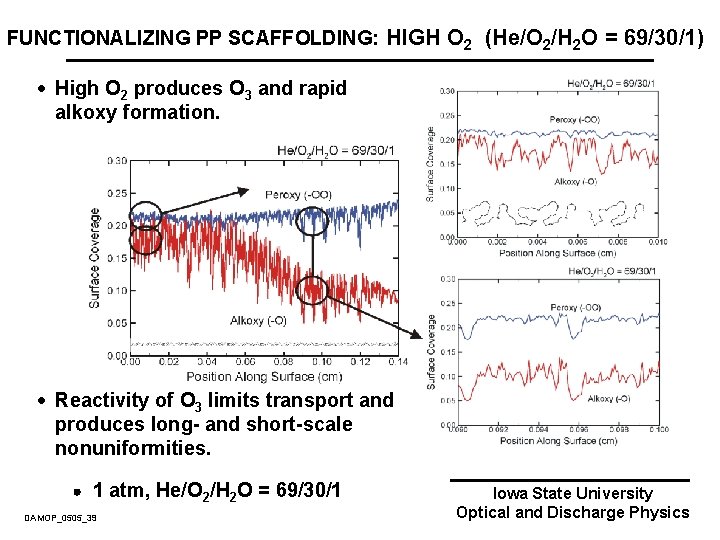
FUNCTIONALIZING PP SCAFFOLDING: HIGH O 2 (He/O 2/H 2 O = 69/30/1) · High O 2 produces O 3 and rapid alkoxy formation. · Reactivity of O 3 limits transport and produces long- and short-scale nonuniformities. 1 atm, He/O 2/H 2 O = 69/30/1 DAMOP_0505_39 Iowa State University Optical and Discharge Physics

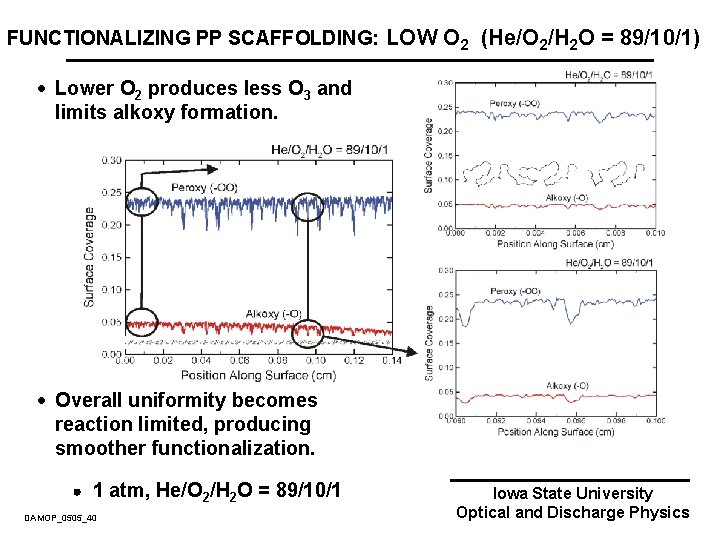
FUNCTIONALIZING PP SCAFFOLDING: LOW O 2 (He/O 2/H 2 O = 89/10/1) · Lower O 2 produces less O 3 and limits alkoxy formation. · Overall uniformity becomes reaction limited, producing smoother functionalization. 1 atm, He/O 2/H 2 O = 89/10/1 DAMOP_0505_40 Iowa State University Optical and Discharge Physics

REMINDER: LOCAL STRUCTURE MATTERS · The reactivity of =C-H to gas phase species depends and with other surface species on their local bonding and orientation on surface. · Experimental evidence suggest reactivity scales as: HTERTIARY > HSECONDARY > HPRIMARY 1 atm, N 2/O 2/H 2 O = 79. 5/1 DAMOP_0505_41 Iowa State University Optical and Discharge Physics
![COVERAGE OF PEROXY COO BY BONDING AT 10 ms Primary and secondary COVERAGE OF PEROXY [=C-O-O ] BY BONDING AT 10 ms · Primary and secondary](https://slidetodoc.com/presentation_image_h/9e3d6d3858d5806dcf6f7ced011e759c/image-43.jpg)
COVERAGE OF PEROXY [=C-O-O ] BY BONDING AT 10 ms · Primary and secondary sites with large view angles are rapidly functionalized to peroxy. · Alkyl tertiary sites lag and are susceptible to OH, O 3 passivation 1 atm, N 2/O 2/H 2 O = 79. 5/1 DAMOP_0505_42 Iowa State University Optical and Discharge Physics
![COVERAGE OF PEROXY COO BY BONDING AT 140 ms Long term production COVERAGE OF PEROXY [=C-O-O ] BY BONDING AT 140 ms · Long term production](https://slidetodoc.com/presentation_image_h/9e3d6d3858d5806dcf6f7ced011e759c/image-44.jpg)
COVERAGE OF PEROXY [=C-O-O ] BY BONDING AT 140 ms · Long term production of O 3 and reactions between surface species favor secondary and tertiary sites. · Uniformity improves (mostly). 1 atm, N 2/O 2/H 2 O = 79. 5/1 DAMOP_0505_43 Iowa State University Optical and Discharge Physics

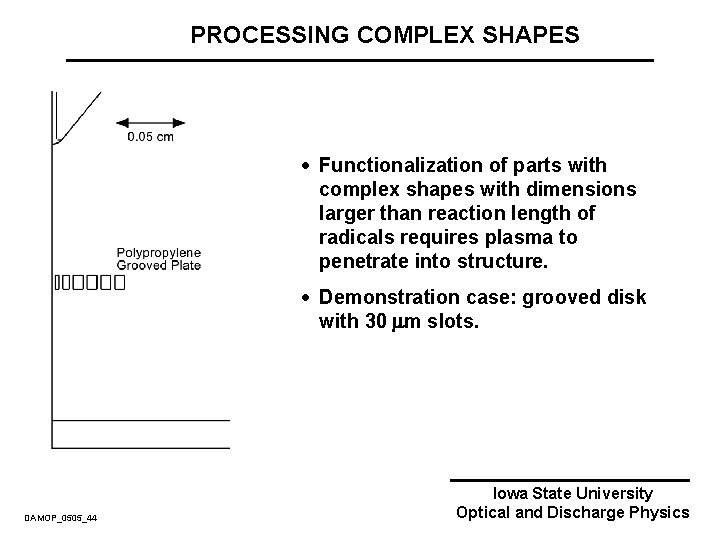
PROCESSING COMPLEX SHAPES · Functionalization of parts with complex shapes with dimensions larger than reaction length of radicals requires plasma to penetrate into structure. · Demonstration case: grooved disk with 30 m slots. DAMOP_0505_44 Iowa State University Optical and Discharge Physics

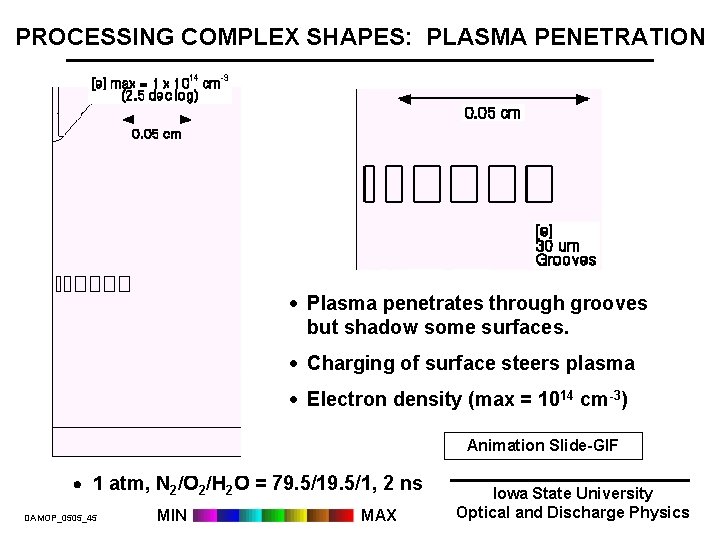
PROCESSING COMPLEX SHAPES: PLASMA PENETRATION · Plasma penetrates through grooves but shadow some surfaces. · Charging of surface steers plasma · Electron density (max = 1014 cm-3) Animation Slide-GIF 1 atm, N 2/O 2/H 2 O = 79. 5/1, 2 ns DAMOP_0505_45 MIN MAX Iowa State University Optical and Discharge Physics

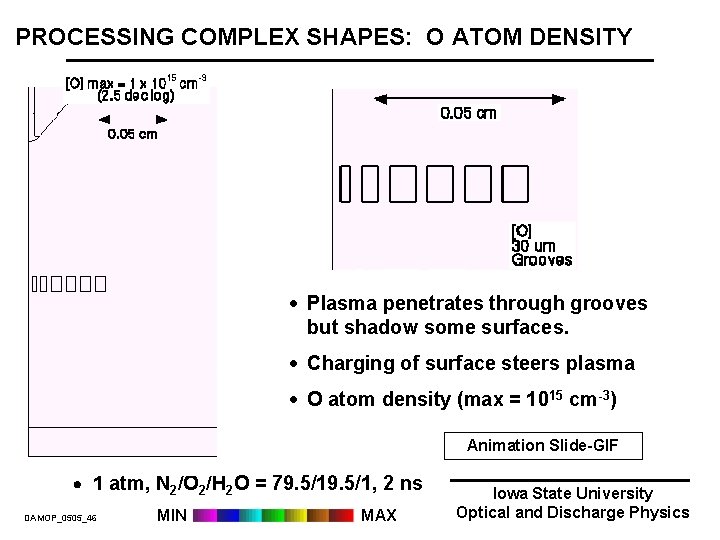
PROCESSING COMPLEX SHAPES: O ATOM DENSITY · Plasma penetrates through grooves but shadow some surfaces. · Charging of surface steers plasma · O atom density (max = 1015 cm-3) Animation Slide-GIF 1 atm, N 2/O 2/H 2 O = 79. 5/1, 2 ns DAMOP_0505_46 MIN MAX Iowa State University Optical and Discharge Physics

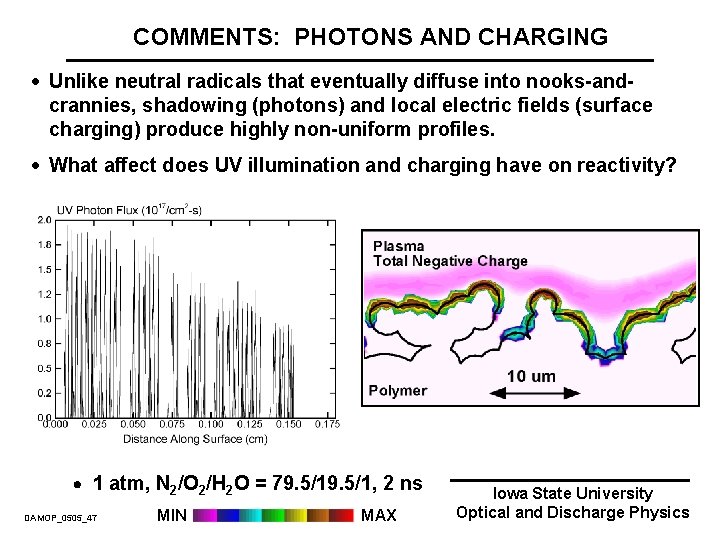
COMMENTS: PHOTONS AND CHARGING · Unlike neutral radicals that eventually diffuse into nooks-andcrannies, shadowing (photons) and local electric fields (surface charging) produce highly non-uniform profiles. · What affect does UV illumination and charging have on reactivity? 1 atm, N 2/O 2/H 2 O = 79. 5/1, 2 ns DAMOP_0505_47 MIN MAX Iowa State University Optical and Discharge Physics

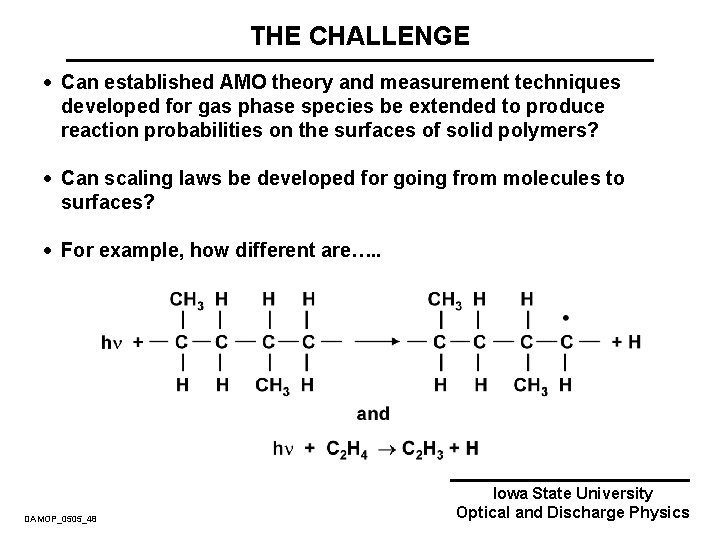
THE CHALLENGE · Can established AMO theory and measurement techniques developed for gas phase species be extended to produce reaction probabilities on the surfaces of solid polymers? · Can scaling laws be developed for going from molecules to surfaces? · For example, how different are…. . DAMOP_0505_48 Iowa State University Optical and Discharge Physics

OPPORTUNITIES AND CONCLUDING REMARKS · The interaction of plasma produced species with polymer surfaces is an exceedingly rich field of study. · The are very (very [very]) few fundamental studies capable of producing reaction probabilities of even simple systems such as O atoms on polypropylene or polyethylene. · Probabilities for reactions between surface species are only now becoming quantified. (Session C 1: “Interaction of Slow Electrons with Biomolecules”) · Photon and charging effects on rates……unknown. · Improving our fundamental understanding and predictive capability (and leveraging commodity techniques) will revolutionize fields such as health products. DAMOP_0505_49 Iowa State University Optical and Discharge Physics