Planning an Investigation 1 of 36 Boardworks Ltd

Planning an Investigation 1 of 36 © Boardworks Ltd 2011

2 of 36 © Boardworks Ltd 2011

Planning an investigation As part of your GCSE Science course you will be assessed on your ability to plan, collect results, analyse the data, draw conclusions and evaluate investigations. You will be expected to identify the variables in investigations and to explain how to collect reliable results that will allow you to draw valid conclusions. What should you consider when planning an investigation? 3 of 36 © Boardworks Ltd 2011

Deciding what to investigate Scientists carry out experiments to test a hypothesis. A hypothesis is an idea that could explain an observation. It can be tested by doing an investigation. For example, one hypothesis could be: ‘Drinking caffeine makes you more alert. ’ The first thing to decide is how to measure how alert someone is. If they are alert, their reactions should be fast. Coffee contains caffeine, so you could measure the effect coffee has on their reaction times. 4 of 36 © Boardworks Ltd 2011

Deciding what to investigate Jack notices the effects of acid rain on marble statues and decides to investigate the reaction between hydrochloric acid and marble chips. What specifically could he investigate? Jack knows the reaction produces carbon dioxide, so he could investigate how acid concentration affects the rate at which it is produced. Ca. CO 3 + 2 HCl Ca. Cl 2 + CO 2 + H 2 O What could his hypothesis be? “Carbon dioxide is produced faster when marble chips react with more concentrated acid. ” 5 of 36 © Boardworks Ltd 2011
Deciding what to investigate Samira has been reading about the use of thermistors in thermostats and decides to investigate the link between temperature and current. What could her hypothesis be? “The current through a thermistor increases as its temperature decreases. ” 6 of 36 © Boardworks Ltd 2011
What should be investigated? 7 of 36 © Boardworks Ltd 2011

8 of 36 © Boardworks Ltd 2011
Types of variable A variable is a quantity or characteristic that can take different values. Every investigation involves three sorts of variable: l an independent variable, which is changed to test a hypothesis l a dependent variable, which is measured to show the effect of this change l control variables, which must be kept the same to make it a fair test. 9 of 36 © Boardworks Ltd 2011
What’s the variable (1)? 10 of 36 © Boardworks Ltd 2011

More types of variable The main types of variable are: l categoric variables, which can be described with words, like eye colour l continuous variables, which can have any value between certain limits, like the temperature of water. There also: l discrete variables, which can only be whole numbers, like the number of insects l ordered variables, which can be ranked, like large, medium or small marble chips. 11 of 36 © Boardworks Ltd 2011
What’s the variable (2)? 12 of 36 © Boardworks Ltd 2011

13 of 36 © Boardworks Ltd 2011
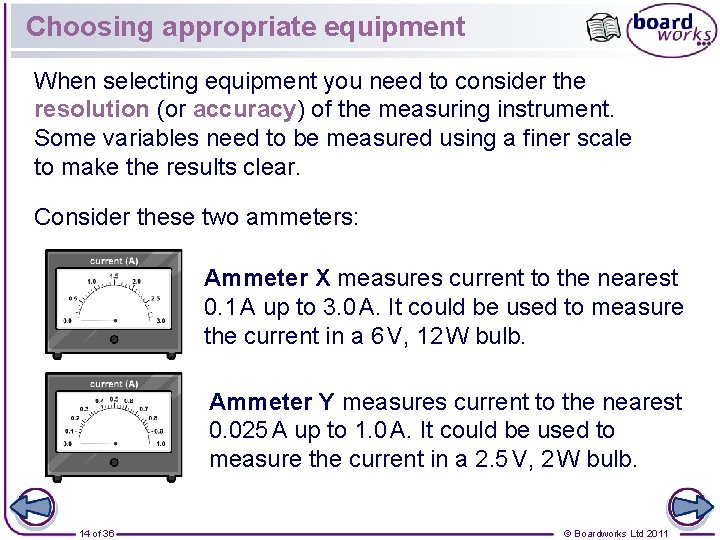
Choosing appropriate equipment When selecting equipment you need to consider the resolution (or accuracy) of the measuring instrument. Some variables need to be measured using a finer scale to make the results clear. Consider these two ammeters: Ammeter X measures current to the nearest 0. 1 A up to 3. 0 A. It could be used to measure the current in a 6 V, 12 W bulb. Ammeter Y measures current to the nearest 0. 025 A up to 1. 0 A. It could be used to measure the current in a 2. 5 V, 2 W bulb. 14 of 36 © Boardworks Ltd 2011

Choosing appropriate equipment To measure the volume of acid needed to neutralize an alkali, a small volume of acid needs to be measured accurately. A burette is the best instrument to use. It can measure up to 50 cm 3 to the nearest 0. 1 cm 3. In an investigation to measure the time taken for a given length of magnesium ribbon to completely react in excess hydrochloric acid, a 100 cm 3 measuring cylinder that measures to the nearest 2 cm 3 is good enough. 15 of 36 © Boardworks Ltd 2011
What equipment should be used? 16 of 36 © Boardworks Ltd 2011

17 of 36 © Boardworks Ltd 2011
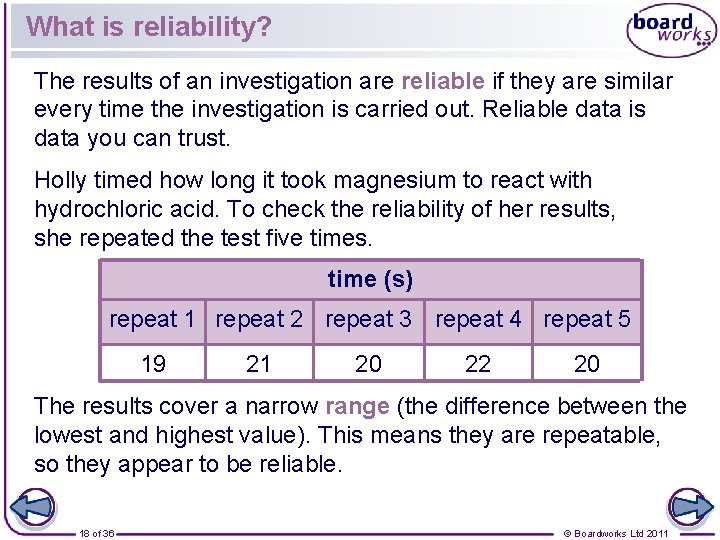
What is reliability? The results of an investigation are reliable if they are similar every time the investigation is carried out. Reliable data is data you can trust. Holly timed how long it took magnesium to react with hydrochloric acid. To check the reliability of her results, she repeated the test five times. time (s) repeat 1 repeat 2 repeat 3 repeat 4 repeat 5 19 21 20 22 20 The results cover a narrow range (the difference between the lowest and highest value). This means they are repeatable, so they appear to be reliable. 18 of 36 © Boardworks Ltd 2011
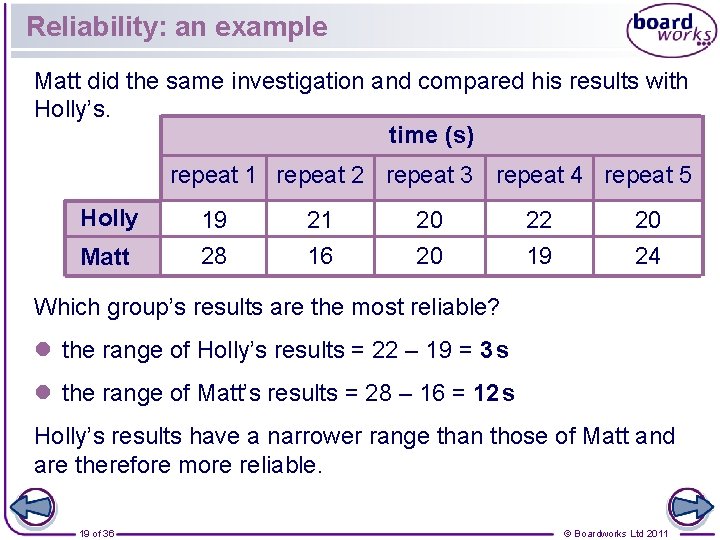
Reliability: an example Matt did the same investigation and compared his results with Holly’s. time (s) repeat 1 repeat 2 repeat 3 repeat 4 repeat 5 Holly Matt 19 28 21 16 20 20 22 19 20 24 Which group’s results are the most reliable? l the range of Holly’s results = 22 – 19 = 3 s l the range of Matt’s results = 28 – 16 = 12 s Holly’s results have a narrower range than those of Matt and are therefore more reliable. 19 of 36 © Boardworks Ltd 2011
Reliability question 20 of 36 © Boardworks Ltd 2011
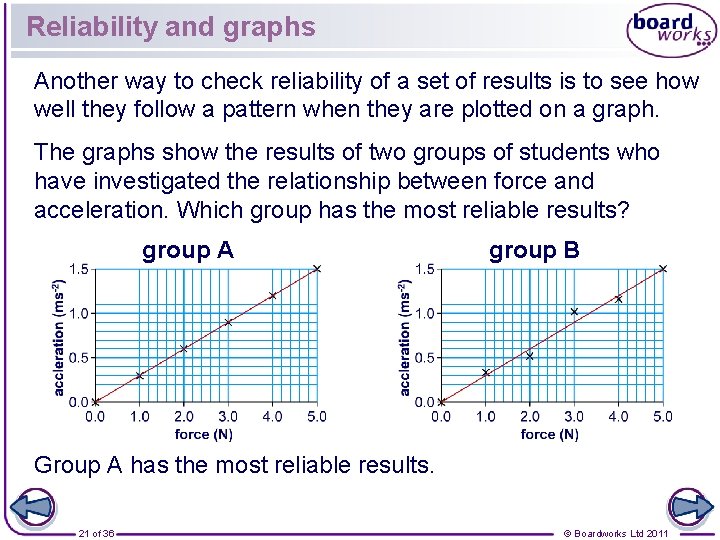
Reliability and graphs Another way to check reliability of a set of results is to see how well they follow a pattern when they are plotted on a graph. The graphs show the results of two groups of students who have investigated the relationship between force and acceleration. Which group has the most reliable results? group A group B Group A has the most reliable results. 21 of 36 © Boardworks Ltd 2011
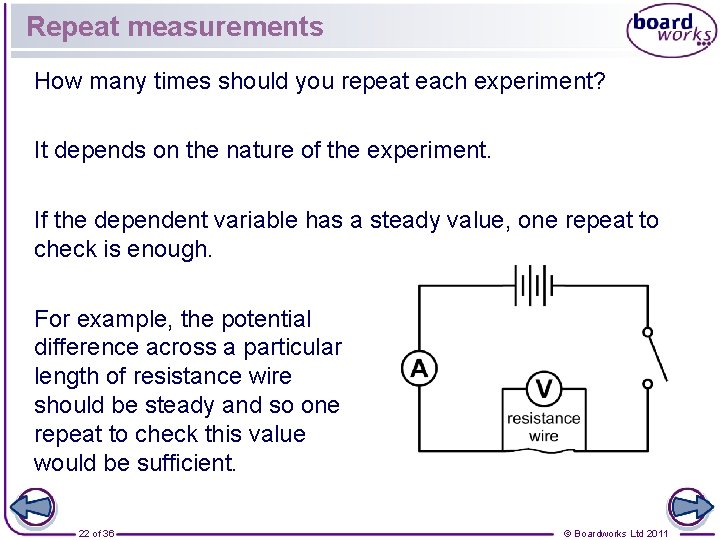
Repeat measurements How many times should you repeat each experiment? It depends on the nature of the experiment. If the dependent variable has a steady value, one repeat to check is enough. For example, the potential difference across a particular length of resistance wire should be steady and so one repeat to check this value would be sufficient. 22 of 36 © Boardworks Ltd 2011
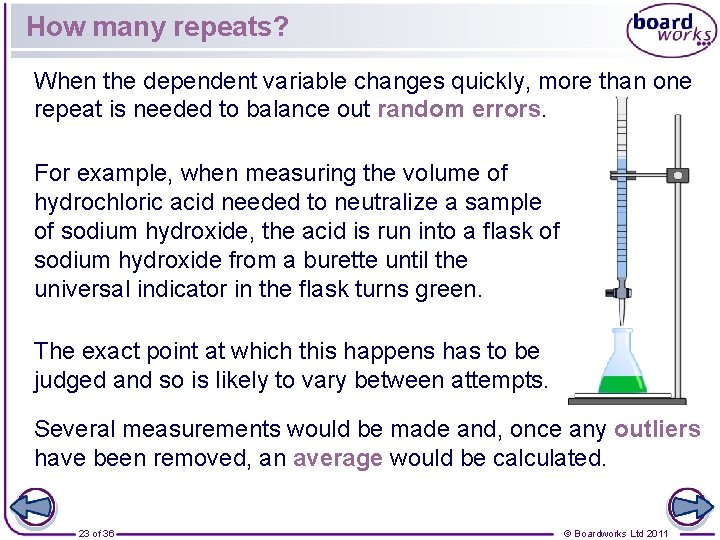
How many repeats? When the dependent variable changes quickly, more than one repeat is needed to balance out random errors. For example, when measuring the volume of hydrochloric acid needed to neutralize a sample of sodium hydroxide, the acid is run into a flask of sodium hydroxide from a burette until the universal indicator in the flask turns green. The exact point at which this happens has to be judged and so is likely to vary between attempts. Several measurements would be made and, once any outliers have been removed, an average would be calculated. 23 of 36 © Boardworks Ltd 2011
One or multiple repeats? 24 of 36 © Boardworks Ltd 2011
What is validity? 25 of 36 © Boardworks Ltd 2011
Ensuring validity What makes conclusions valid? l They must be based on a fair test – only the independent variable must changed. The others should be controlled. l You must test as many values of the independent variable as possible, over as wide a range as possible, and the intervals between them must not be too big. l Any uncertainty in a measurement must be as small as possible compared to the size of the measurement itself. l Measuring instruments must be well calibrated and working properly. 26 of 36 © Boardworks Ltd 2011
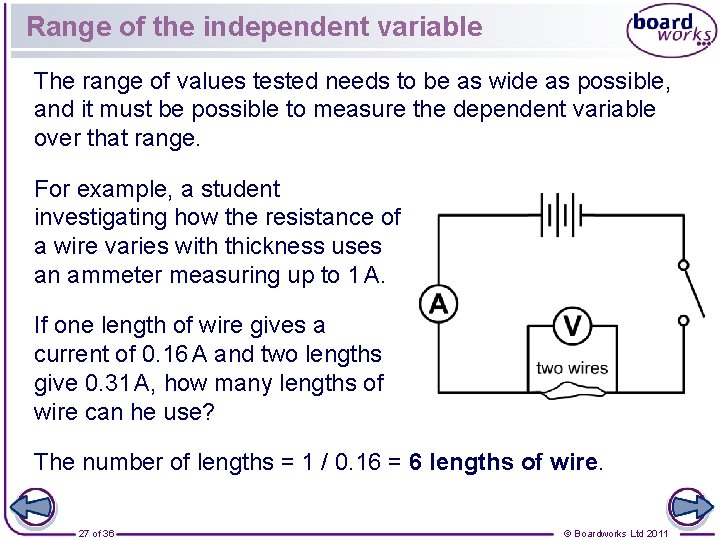
Range of the independent variable The range of values tested needs to be as wide as possible, and it must be possible to measure the dependent variable over that range. For example, a student investigating how the resistance of a wire varies with thickness uses an ammeter measuring up to 1 A. If one length of wire gives a current of 0. 16 A and two lengths give 0. 31 A, how many lengths of wire can he use? The number of lengths = 1 / 0. 16 = 6 lengths of wire. 27 of 36 © Boardworks Ltd 2011
Choosing an appropriate range 28 of 36 © Boardworks Ltd 2011

29 of 36 © Boardworks Ltd 2011

Risks and hazards Whenever your teacher gives you an experiment to do they carry out a risk assessment beforehand. When designing your own experiment, this becomes your responsibility. This involves identifying hazards and what safety precautions should be taken to reduce the risk of an accident. l A hazard is a possible danger or threat (e. g. HCl is hazardous because it is toxic and corrosive). l Risk is the probability of injury. The risk involved when using HCl can be reduced by wearing safety goggles. 30 of 36 © Boardworks Ltd 2011

Risk assessment: hazard symbols A student is preparing a sample of copper chloride by adding an excess of copper oxide to hydrochloric acid, filtering out the excess copper oxide and then heating the resulting copper chloride solution until it becomes saturated. The bottles of copper oxide and hydrochloric acid have the following hazard symbols on them: copper oxide harmful oxidizing hydrochloric acid toxic corrosive What should the student write in his risk assessment form? 31 of 36 © Boardworks Ltd 2011
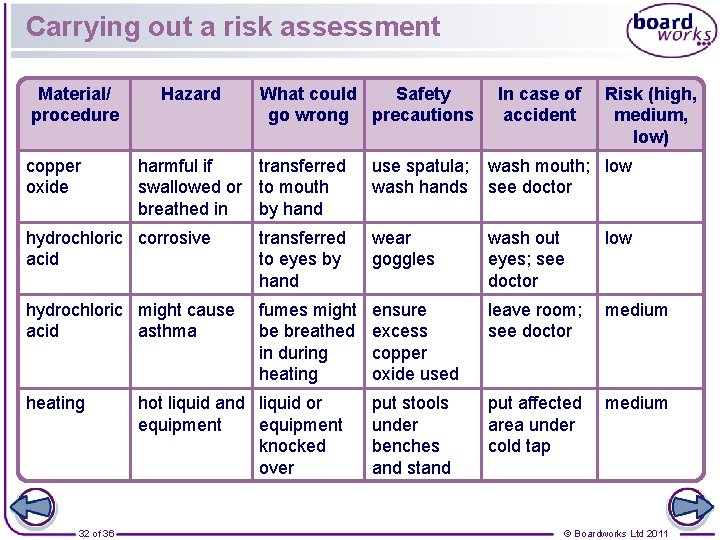
Carrying out a risk assessment Material/ procedure copper oxide Hazard What could Safety go wrong precautions harmful if transferred swallowed or to mouth breathed in by hand In case of accident Risk (high, medium, low) use spatula; wash hands wash mouth; low see doctor hydrochloric corrosive acid transferred to eyes by hand wear goggles wash out eyes; see doctor low hydrochloric might cause acid asthma fumes might be breathed in during heating ensure excess copper oxide used leave room; see doctor medium put stools under benches and stand put affected area under cold tap medium heating 32 of 36 hot liquid and liquid or equipment knocked over © Boardworks Ltd 2011

Writing up experiments (1) An account of an investigation should have certain features. It should start off by including: l the hypothesis being tested or purpose of the investigation l a labelled diagram showing how the apparatus was set up l the range of the independent variable (and the intervals between) and the values measured. What should come next? 33 of 36 © Boardworks Ltd 2011
Writing up experiments (2) The account should also include: l the dependent variable, the equipment used to measure it, the number of repeats, and the steps taken to ensure accuracy l a list of control variables, and an explanation of how their values were controlled l a list of steps that were followed. Where appropriate you should explain how you will take into account the effects of variables that can’t easily be controlled. If you spot problems with your original plan, explain what changes you made and what effect they had. 34 of 36 © Boardworks Ltd 2011
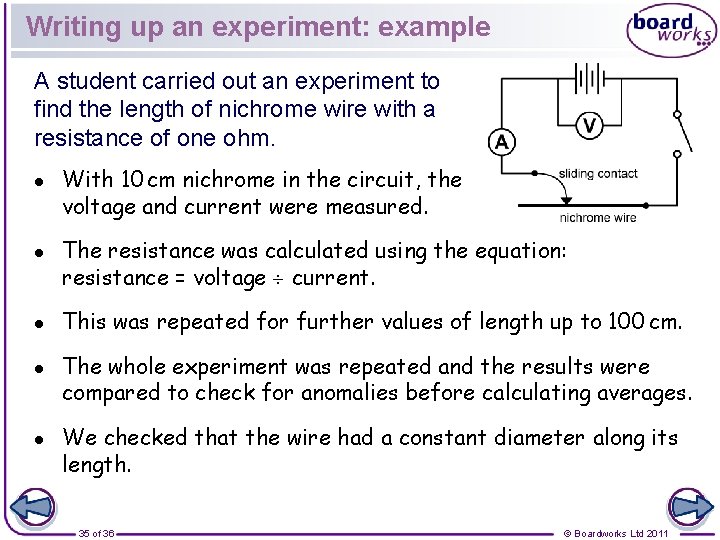
Writing up an experiment: example A student carried out an experiment to find the length of nichrome wire with a resistance of one ohm. l l l With 10 cm nichrome in the circuit, the voltage and current were measured. The resistance was calculated using the equation: resistance = voltage current. This was repeated for further values of length up to 100 cm. The whole experiment was repeated and the results were compared to check for anomalies before calculating averages. We checked that the wire had a constant diameter along its length. 35 of 36 © Boardworks Ltd 2011

Glossary 36 of 36 © Boardworks Ltd 2011
- Slides: 36