1 of 29 Boardworks Ltd 2008 2 of
1 of 29 © Boardworks Ltd 2008
2 of 29 © Boardworks Ltd 2008

Making a revision plan Revising all the science you know can seem like a massive task. The first thing to do is to break down all the material you need to learn into small topics. Next, make a list of the days you can revise, and match a topic to each of these days. This is your revision plan. Tick off each day’s revision when you have completed it. Review topics that you’ve already revised by going over them again quickly the next day. 3 of 29 © Boardworks Ltd 2008

How to revise There are different ways to revise, and everyone has their own favourite way. Try different ways of revising and test yourself to see which works best. However, everyone should revise: l in a quiet place where you won’t be disturbed – no background TV or music! l sitting on a chair at a desk, not slumped on the sofa l in good light – so you don’t strain your eyes l with the correct equipment – paper, pens, pencils, clock, etc. 4 of 29 © Boardworks Ltd 2008

What’s the best revision method for me? When revising it’s very important not to just read through your books – your brain needs to do something with the information. Here are some suggestions for active revision: l Write short notes on key ideas. l Draw a concept map, linking key words and ideas together. l Record your notes as an MP 3 file and play them back. l Write key words on Post-Its and stick them around the house. l Draw pictures/diagrams to illustrate an idea. 5 of 29 © Boardworks Ltd 2008

When should you revise? Start revising early in the morning, and don’t work for more than two hours at a time. Use a clock and be strict about stopping, even if you’re in the middle of something. Take a five minute break every 25 minutes. You learn more effectively at the start and end of a revision session, so it’s better to have several shorter sessions than one long session. Give yourself a reward after the two hours is up, such as listening to a favourite song, watching a TV programme, or playing outside. 6 of 29 © Boardworks Ltd 2008
What’s the best revision method for me? 7 of 29 © Boardworks Ltd 2008
8 of 29 © Boardworks Ltd 2008
Key ideas in KS 3 chemistry All the chemistry you have studied is based on four key ideas: 1. Everything is made of particles. 2. Elements form compounds in chemical reactions. 3. Elements and compounds have characteristic patterns of reactivity. 4. Geological activity is caused by chemical and physical processes. 9 of 29 © Boardworks Ltd 2008
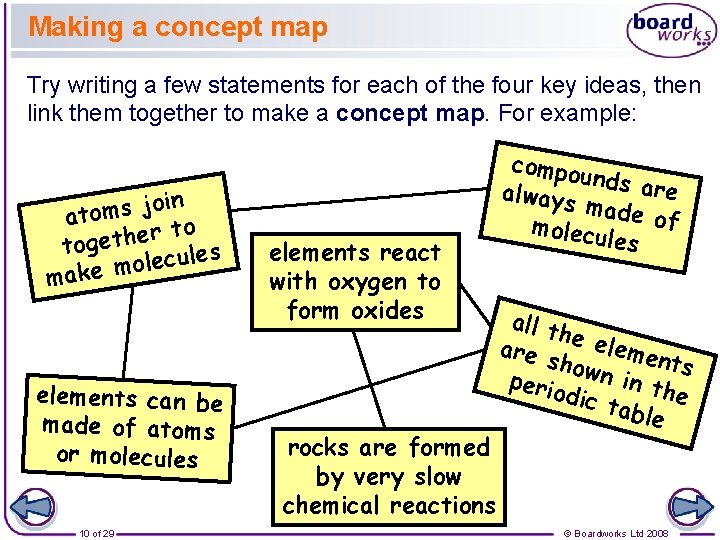
Making a concept map Try writing a few statements for each of the four key ideas, then link them together to make a concept map. For example: n i o j s m ato o t r e h t toge ules c e l o m make elements can be made of atoms or molecules 10 of 29 elements react with oxygen to form oxides rocks are formed by very slow chemical reactions compou n always ds are made of molecu les all t h are e eleme nts show perio n in th e dic t able © Boardworks Ltd 2008

Preparing for the exam Have a good night’s sleep before the day of the exam so you wake up fresh and ready to go. Eat a good breakfast. Studies show that students who eat a healthy breakfast perform better than those who do not. Make sure you have the following equipment ready the night before the exam: l two pens (black or blue) l a 30 cm ruler l two pencils l a protractor l a rubber and pencil sharpener l a calculator. 11 of 29 © Boardworks Ltd 2008

In the exam While you’re waiting for the exam to start, read everything on the front cover of the exam paper. When the exam starts, read each question carefully before you start writing and check how many marks it is worth. Pace yourself so you don’t run out of time. Aim for 3– 4 minutes per question. If you get stuck on a question, skip it and come back to it at the end. Always try to answer every part of every question – it’s better to put something down than to leave your answer blank. 12 of 29 © Boardworks Ltd 2008

Answering questions Top tips on how to maximize your chances of top marks: l Write as clearly and neatly as you can. l Questions that start with ‘Explain…’ or ‘Describe…’ usually need longer answers than questions that start with ‘List…’ or ‘State…’, but don’t waffle. l If you are asked to calculate something, show your working out – even if the final answer is wrong, you may still get some marks. l Remember to add units to calculations and graphs if the question asks for them, and add labels to diagrams. l If a question says ‘Tick one box’, or ‘Draw two lines’, do it! 13 of 29 © Boardworks Ltd 2008

Answering questions Not everything in the exams will test your memory. Sometimes you will be given information about chemical reactions or substances you have never heard of. Don’t worry! The question is trying to see if you can analyse information and work out the important facts from it. For example, adding solid samarium powder to a solution of platinum chloride causes the displacement of platinum. What does this tell you about the reactivity of samarium and platinum? No-one would expect you to know about samarium and platinum before the exam, but read the question carefully, consider what you already know about reactivity of metals and you should be able to answer it. 14 of 29 © Boardworks Ltd 2008

Identifying poor answers 15 of 29 © Boardworks Ltd 2008
16 of 29 © Boardworks Ltd 2008

Worked sample question 1 1. Railway lines can be joined together by pouring molten iron into the gap between them. (a) The molten iron is produced by reacting aluminium with iron oxide. Complete the word equation for the reaction: 1 mark aluminium + iron → iron + aluminium oxide The aluminium displaces the iron from the iron oxide because aluminium is more reactive than iron. This means that aluminium oxide is formed. 17 of 29 © Boardworks Ltd 2008
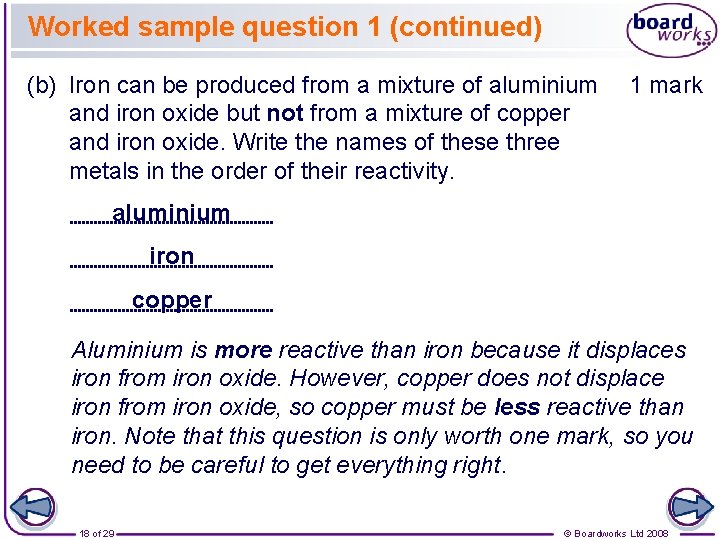
Worked sample question 1 (continued) (b) Iron can be produced from a mixture of aluminium and iron oxide but not from a mixture of copper and iron oxide. Write the names of these three metals in the order of their reactivity. 1 mark aluminium iron copper Aluminium is more reactive than iron because it displaces iron from iron oxide. However, copper does not displace iron from iron oxide, so copper must be less reactive than iron. Note that this question is only worth one mark, so you need to be careful to get everything right. 18 of 29 © Boardworks Ltd 2008
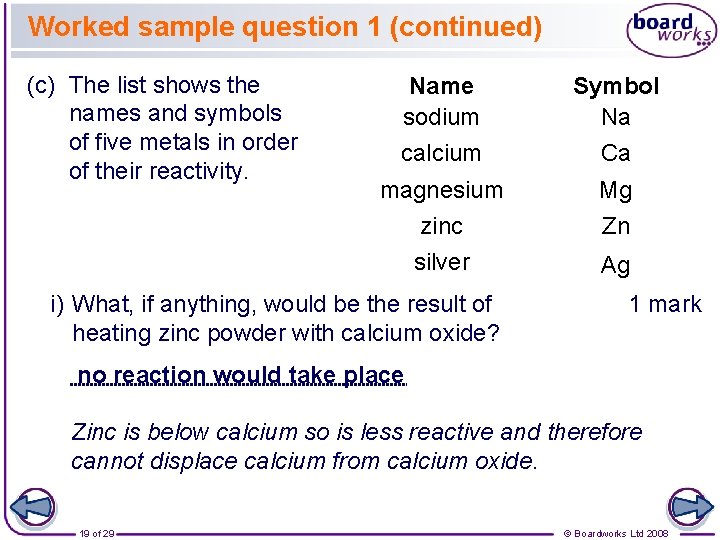
Worked sample question 1 (continued) (c) The list shows the names and symbols of five metals in order of their reactivity. Name sodium Symbol Na calcium Ca magnesium zinc Mg Zn silver Ag i) What, if anything, would be the result of heating zinc powder with calcium oxide? 1 mark no reaction would take place Zinc is below calcium so is less reactive and therefore cannot displace calcium from calcium oxide. 19 of 29 © Boardworks Ltd 2008
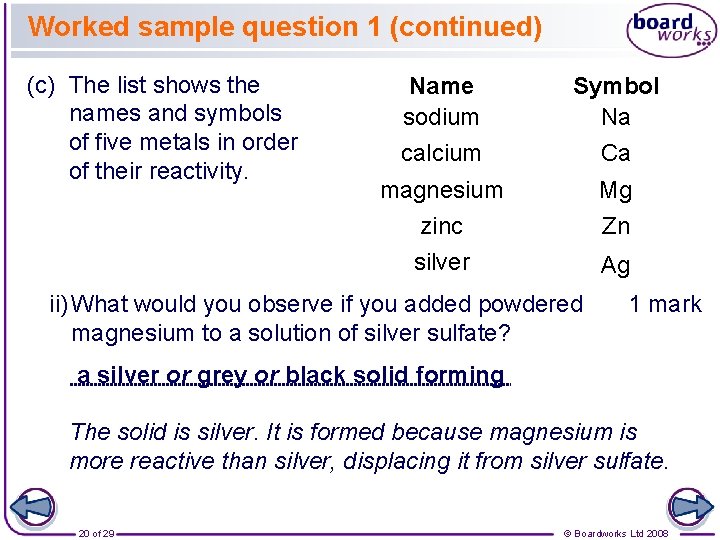
Worked sample question 1 (continued) (c) The list shows the names and symbols of five metals in order of their reactivity. Name sodium Symbol Na calcium Ca magnesium zinc Mg Zn silver Ag ii) What would you observe if you added powdered magnesium to a solution of silver sulfate? 1 mark a silver or grey or black solid forming The solid is silver. It is formed because magnesium is more reactive than silver, displacing it from silver sulfate. 20 of 29 © Boardworks Ltd 2008
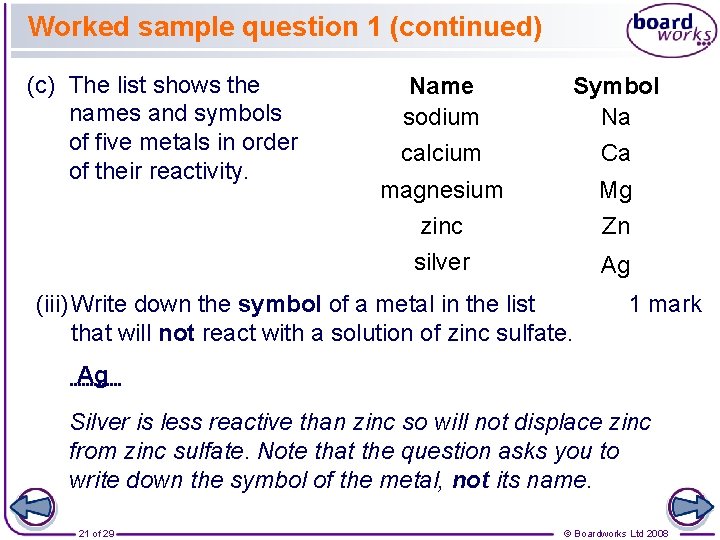
Worked sample question 1 (continued) (c) The list shows the names and symbols of five metals in order of their reactivity. Name sodium Symbol Na calcium Ca magnesium zinc Mg Zn silver Ag (iii) Write down the symbol of a metal in the list that will not react with a solution of zinc sulfate. 1 mark Ag Silver is less reactive than zinc so will not displace zinc from zinc sulfate. Note that the question asks you to write down the symbol of the metal, not its name. 21 of 29 © Boardworks Ltd 2008
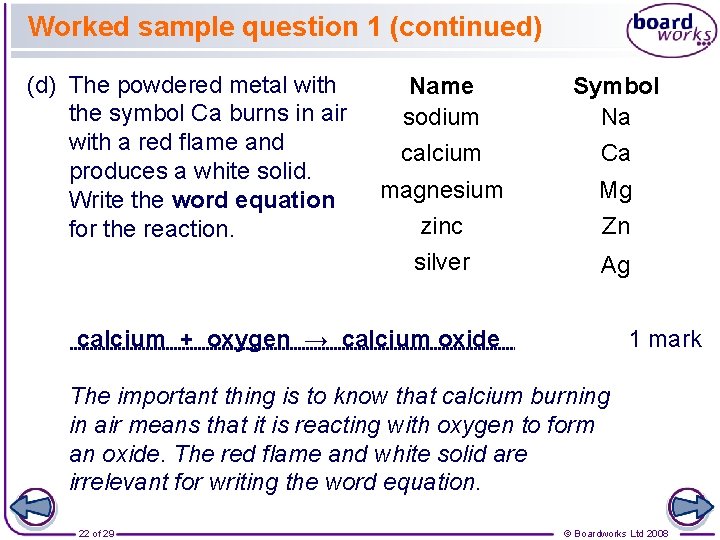
Worked sample question 1 (continued) (d) The powdered metal with the symbol Ca burns in air with a red flame and produces a white solid. Write the word equation for the reaction. Name sodium Symbol Na calcium Ca magnesium zinc Mg Zn silver Ag calcium + oxygen → calcium oxide 1 mark The important thing is to know that calcium burning in air means that it is reacting with oxygen to form an oxide. The red flame and white solid are irrelevant for writing the word equation. 22 of 29 © Boardworks Ltd 2008
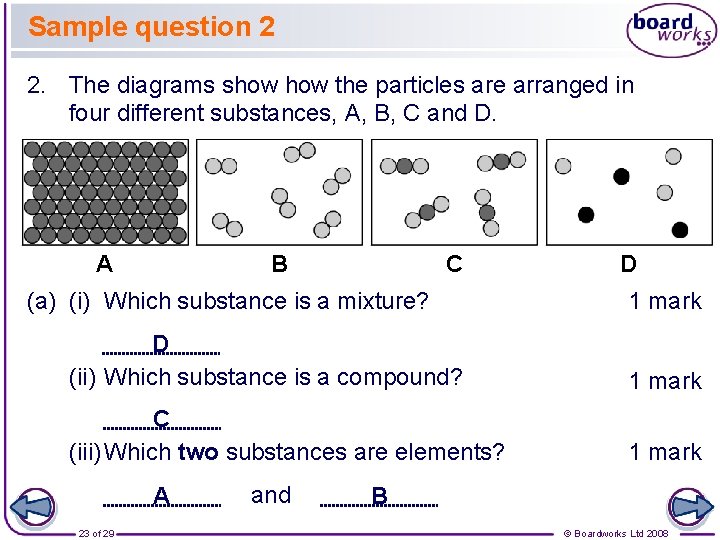
Sample question 2 2. The diagrams show the particles are arranged in four different substances, A, B, C and D. A B C (a) (i) Which substance is a mixture? D 1 mark D (ii) Which substance is a compound? 1 mark C (iii) Which two substances are elements? 1 mark A 23 of 29 and B © Boardworks Ltd 2008
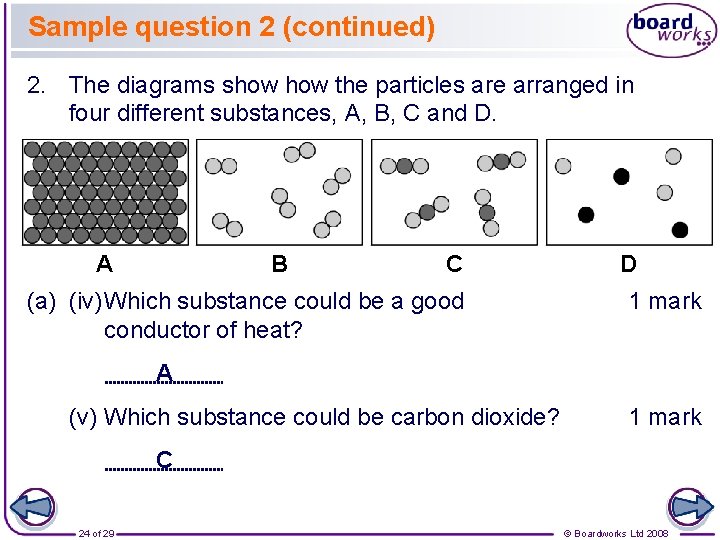
Sample question 2 (continued) 2. The diagrams show the particles are arranged in four different substances, A, B, C and D. A B C (a) (iv)Which substance could be a good conductor of heat? D 1 mark A (v) Which substance could be carbon dioxide? 1 mark C 24 of 29 © Boardworks Ltd 2008
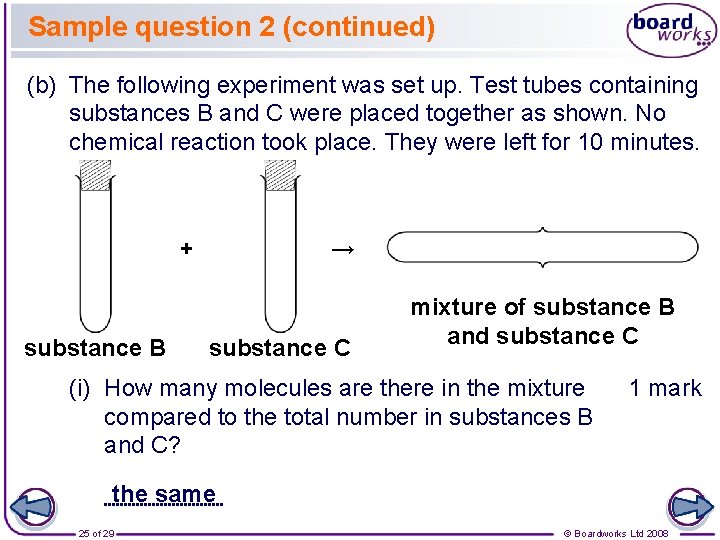
Sample question 2 (continued) (b) The following experiment was set up. Test tubes containing substances B and C were placed together as shown. No chemical reaction took place. They were left for 10 minutes. + substance B → substance C mixture of substance B and substance C (i) How many molecules are there in the mixture compared to the total number in substances B and C? 1 mark the same 25 of 29 © Boardworks Ltd 2008
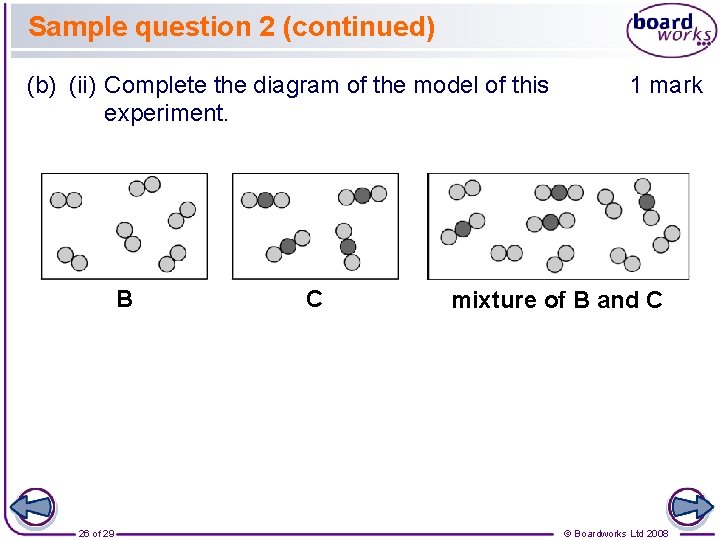
Sample question 2 (continued) (b) (ii) Complete the diagram of the model of this experiment. B 26 of 29 C 1 mark mixture of B and C © Boardworks Ltd 2008
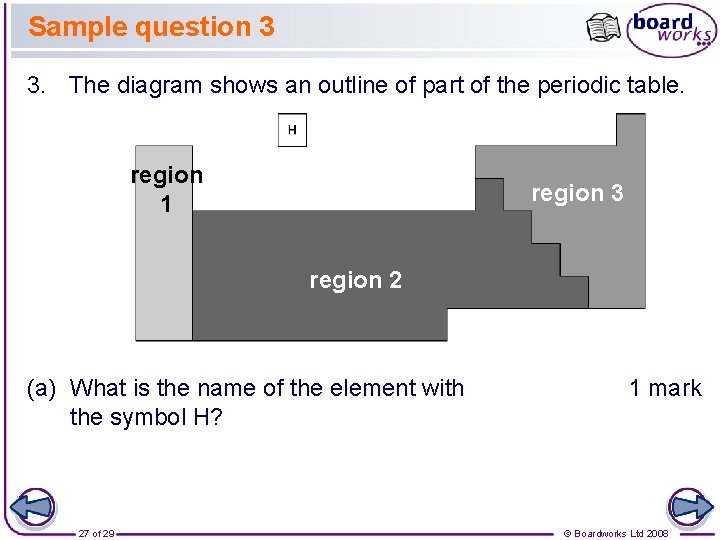
Sample question 3 3. The diagram shows an outline of part of the periodic table. region 1 region 3 region 2 (a) What is the name of the element with the symbol H? 27 of 29 1 mark © Boardworks Ltd 2008
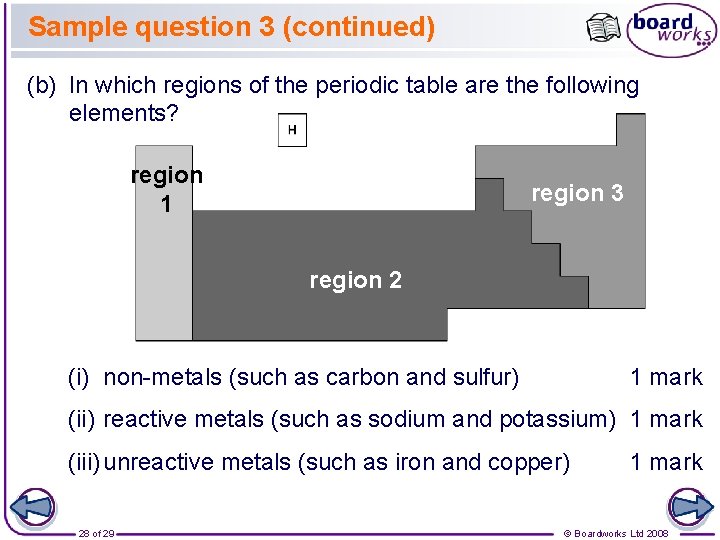
Sample question 3 (continued) (b) In which regions of the periodic table are the following elements? region 1 region 3 region 2 (i) non-metals (such as carbon and sulfur) 1 mark (ii) reactive metals (such as sodium and potassium) 1 mark (iii) unreactive metals (such as iron and copper) 28 of 29 1 mark © Boardworks Ltd 2008
Sample question 3 (continued) (c) Why is copper sulfate not shown in the periodic table? 29 of 29 1 mark © Boardworks Ltd 2008
- Slides: 29