Pharmacophores in Chemoinformatics 1 Pharmacophore Patterns Topological Fingerprints



![Exploiting pharmacophore patterns… • N-dimensional vector D(M)=[D 1(M), D 2(M), …, DN(M)]; each Di Exploiting pharmacophore patterns… • N-dimensional vector D(M)=[D 1(M), D 2(M), …, DN(M)]; each Di](https://slidetodoc.com/presentation_image/7cefd722a8410be95db9b1bd9bb39dc7/image-9.jpg)








- Slides: 29

Pharmacophores in Chemoinformatics: 1. Pharmacophore Patterns & Topological Fingerprints Dragos Horvath Laboratoire d’Info. Chimie UMR 7177 CNRS – Université de Strasbourg horvath@chimie. u-strasbg. fr

The Pharmacophore Way of Life – A Medicinal Chemist’s Dream • (Bio)Molecular Recognition is based on ligand-site interactions of extremely complicated nature – Understanding them requires a solid knowledge of statistical physics and, therefore, of higher maths… – But medicinal chemists hate maths… so they developed a simplified rule set to rationalize ligand binding. • Functional groups of similar physicochemical behavior represent pharmacophore types: – Hydrophobic, Aromatic, Hydrogen Bond (HB) donors, Cations, HB Acceptors, Anions. – Now, we just need to know how each of the six types interacts with the site… welcome to the “pharmacophore” paradigm, farewell higher maths (for the moment, at least)

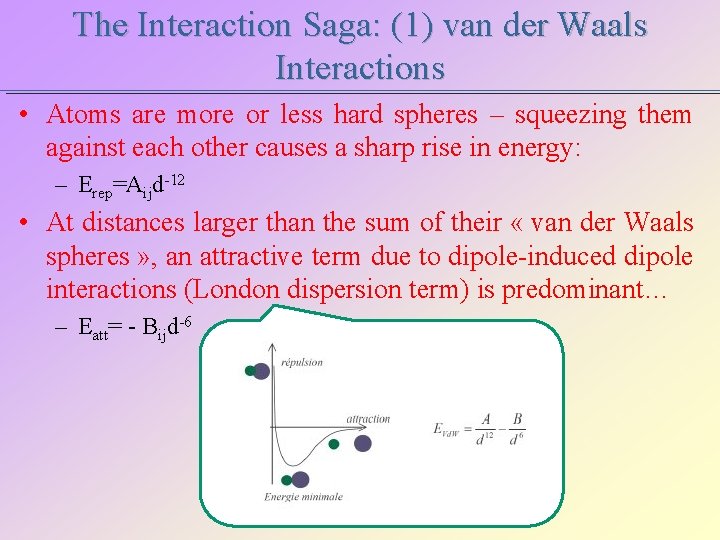
The Interaction Saga: (1) van der Waals Interactions • Atoms are more or less hard spheres – squeezing them against each other causes a sharp rise in energy: – Erep=Aijd-12 • At distances larger than the sum of their « van der Waals spheres » , an attractive term due to dipole-induced dipole interactions (London dispersion term) is predominant… – Eatt= - Bijd-6

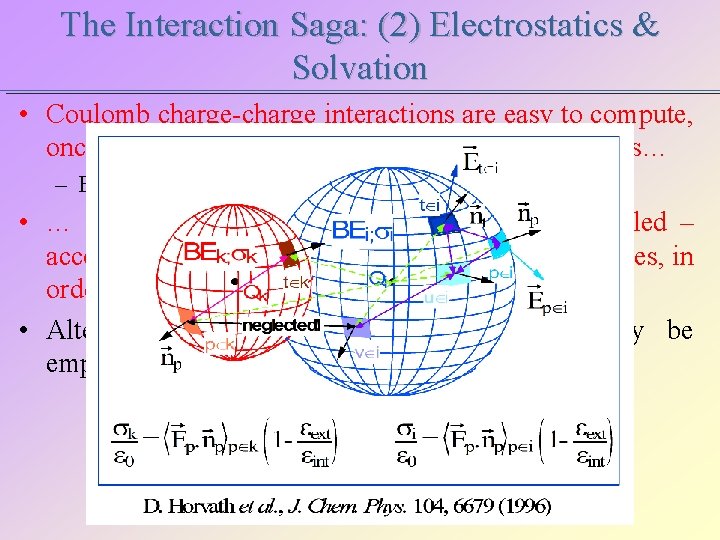
The Interaction Saga: (2) Electrostatics & Solvation • Coulomb charge-charge interactions are easy to compute, once the partial charges Qk are assigned on the atoms… – ECoul=Qi. Qj/4 ped • … and the solvent molecules are explicitly modeled – accountig for all the possible solvation shell structures, in order to estimate a solvation free energy. • Alternatively, a continuum solvent model may be employed.

The Interaction Saga: (2 bis) The Hydrophobic Effect • The mysterious force that separates grease and water is not due to grease-grease van der Waals interactions being stronger than grease-water attraction! • It is not of electrostatic nature either, because greasy alkyl chains have no charges! • Actually, it’s not a force at all, but the consequence of the drift towards a more probable state of matter (? !) • For practical purposes, however, it makes sense to believe that hydrophobes « attract » each other – for making hydrophobic contacts significantly improves binding affinity!

Physical Chemistry For Dummies: The Rules • Hydrophobes make favorable contacts with other hydrophobes (we do not want to know why!). Assume strenght proportional to the buried hydrophobic area. • Hydrophobes in close contact to polar groups cause frustration, for they chase away the water molecules favorably solvating the latter and offer no substitute interactions • Hydrogen bond donors seek to pair with acceptors, so that they may reestablish the water hydrogen bonds they lost • Cations seek to pair with anions and avoid hydrophobes. • Shape is of paramount importance: groups of a same kind may replace each other if they are shaped likely

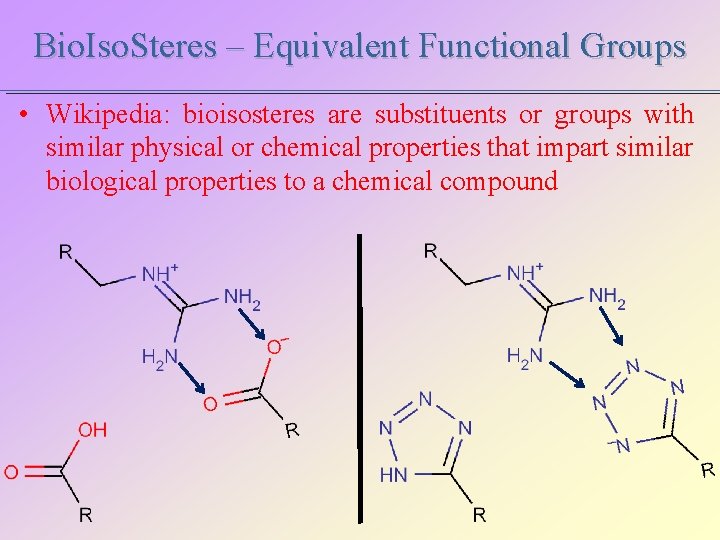
Bio. Iso. Steres – Equivalent Functional Groups • Wikipedia: bioisosteres are substituents or groups with similar physical or chemical properties that impart similar biological properties to a chemical compound

Pharmacophore Patterns • The pharmacophore pattern of a molecule characterizes the relative arrangement of all its pharmacophore types – What pharmacophore types are represented? – How are they arranged (spatially, topologically) with respect to each other ? – How can these aspects be captured numerically to yield molecular descriptors of the pharmacophore pattern? • Note: Pharmacophore patterns are essentially 3 D. Since geometry is determined by connectivity, 2 D “pharmacophore patterns” also make sense!
![Exploiting pharmacophore patterns Ndimensional vector DMD 1M D 2M DNM each Di Exploiting pharmacophore patterns… • N-dimensional vector D(M)=[D 1(M), D 2(M), …, DN(M)]; each Di](https://slidetodoc.com/presentation_image/7cefd722a8410be95db9b1bd9bb39dc7/image-9.jpg)
Exploiting pharmacophore patterns… • N-dimensional vector D(M)=[D 1(M), D 2(M), …, DN(M)]; each Di encodes an element of the pharmacophore pattern – Allows meaningful quantitative definitions of molecular similarity: • Neighborhood Behavior: Similar molecules - characterized by covariant vectors - are likely to display similar biological properties • As chemists do not easily perceive the pharmacophore pattern, such covariance may reveal hidden but real molecular relatedness… – May serve as starting point for searching a binding pharmacophore – the subset of features that really participate in binding to a receptor • Machine learning to select those elements Di that are systematically present in actives, but not in inactives of a molecular learning set!

Some examples of "hidden similarity"

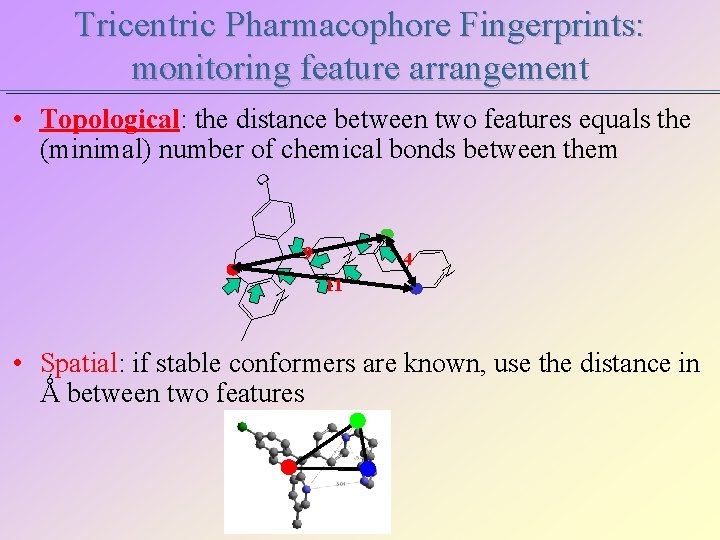
Tricentric Pharmacophore Fingerprints: monitoring feature arrangement • Topological: the distance between two features equals the (minimal) number of chemical bonds between them Cl O N 9 N N 11 4 • Spatial: if stable conformers are known, use the distance in Ǻ between two features

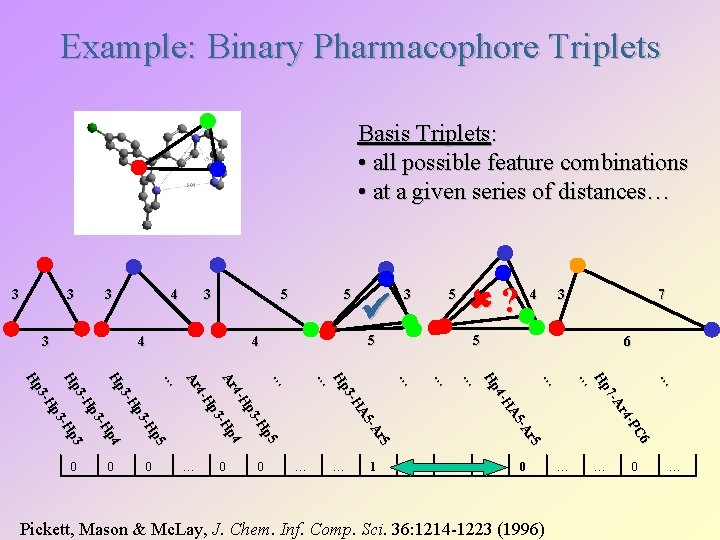
Example: Binary Pharmacophore Triplets Basis Triplets: • all possible feature combinations • at a given series of distances… 3 3 4 3 5 4 0 … … 1 4 3 7 … … 6 … C 6 -P r 4 7 - A Hp … 0 ? … r 5 -A A 5 4 -H Hp … … … 0 5 5 … r 5 -A A 5 3 -H Hp … 0 3 5 4 … p 5 -H p 3 4 -H p 4 Ar -H p 3 4 -H Ar … p 5 -H p 3 3 -H Hp p 4 -H p 3 3 -H p 3 Hp -H p 3 3 -H Hp 0 5 … 0 Pickett, Mason & Mc. Lay, J. Chem. Inf. Comp. Sci. 36: 1214 -1223 (1996) … … 0 …

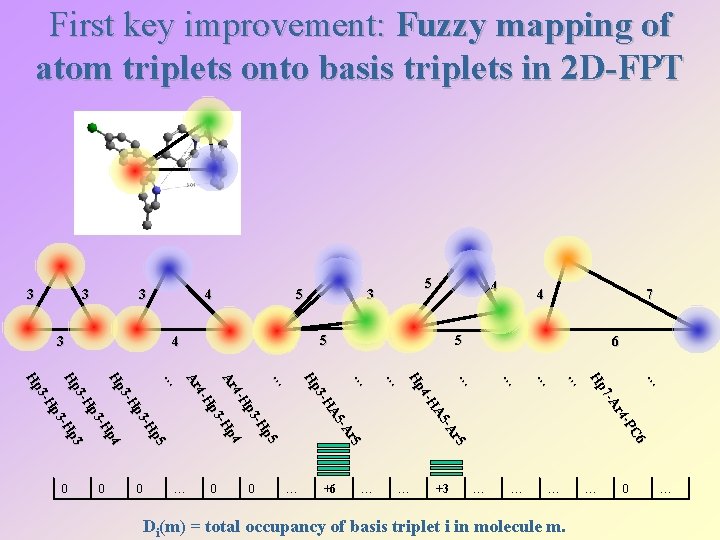
First key improvement: Fuzzy mapping of atom triplets onto basis triplets in 2 D-FPT 3 3 4 4 … +3 … … … Di(m) = total occupancy of basis triplet i in molecule m. … C 6 -P r 4 7 - A Hp … … +6 … … … 0 7 6 … r 5 -A A 5 4 -H Hp 0 … … 4 5 … r 5 -A A 5 3 -H Hp 0 … p 5 -H p 3 4 -H p 4 Ar -H p 3 4 -H Ar 0 5 3 5 4 … p 5 -H p 3 3 -H Hp p 4 -H p 3 3 -H p 3 Hp -H p 3 3 -H Hp 0 5 … 0 …

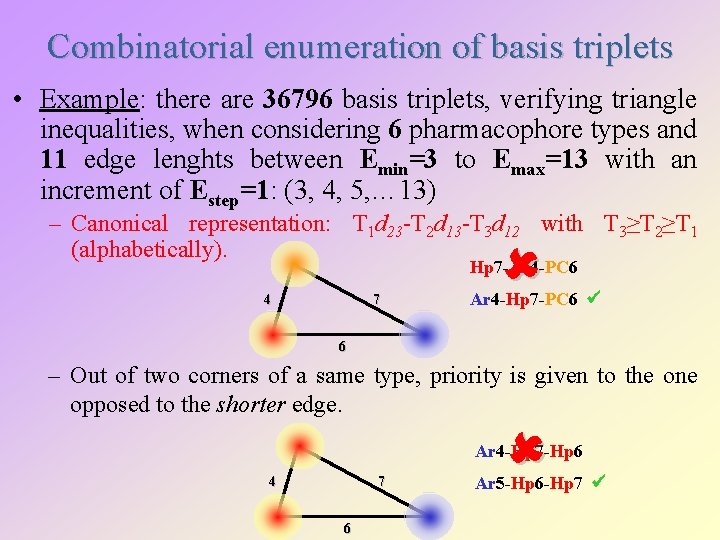
Combinatorial enumeration of basis triplets • Example: there are 36796 basis triplets, verifying triangle inequalities, when considering 6 pharmacophore types and 11 edge lenghts between Emin=3 to Emax=13 with an increment of Estep=1: (3, 4, 5, … 13) – Canonical representation: T 1 d 23 -T 2 d 13 -T 3 d 12 with T 3≥T 2≥T 1 (alphabetically). Hp 7 -Ar 4 -PC 6 4 7 Ar 4 -Hp 7 -PC 6 6 – Out of two corners of a same type, priority is given to the one opposed to the shorter edge. Ar 4 -Hp 7 -Hp 6 4 7 6 Ar 5 -Hp 6 -Hp 7

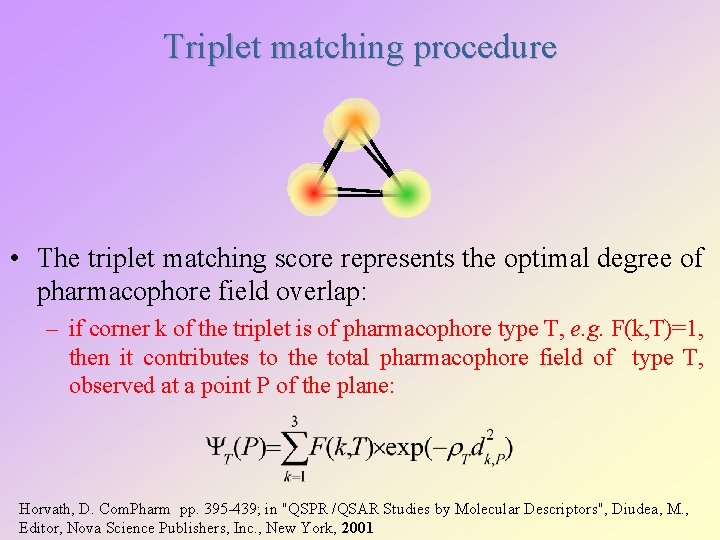
Triplet matching procedure • The triplet matching score represents the optimal degree of pharmacophore field overlap: – if corner k of the triplet is of pharmacophore type T, e. g. F(k, T)=1, then it contributes to the total pharmacophore field of type T, observed at a point P of the plane: Horvath, D. Com. Pharm pp. 395 -439; in "QSPR /QSAR Studies by Molecular Descriptors", Diudea, M. , Editor, Nova Science Publishers, Inc. , New York, 2001

Control parameters for triplet enumeration & matching in two 2 D-FPT versions.

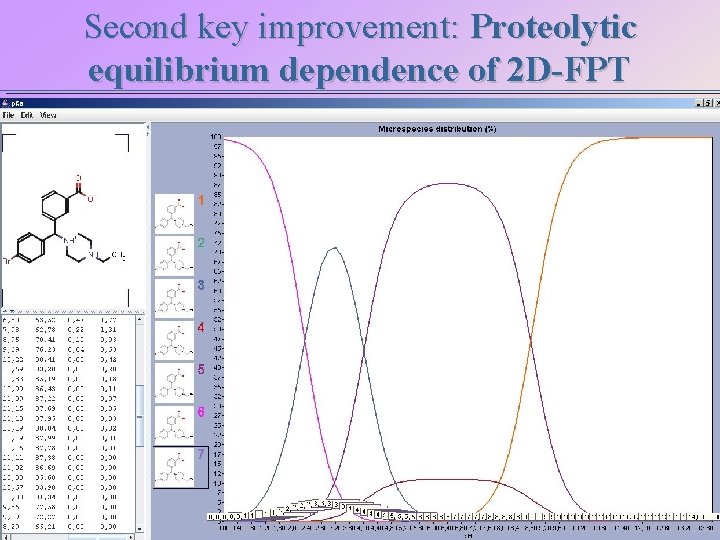
Second key improvement: Proteolytic equilibrium dependence of 2 D-FPT 88% Ar 8 NC 8 PC 8 12% Ar 5 NC 5 PC 8 ?

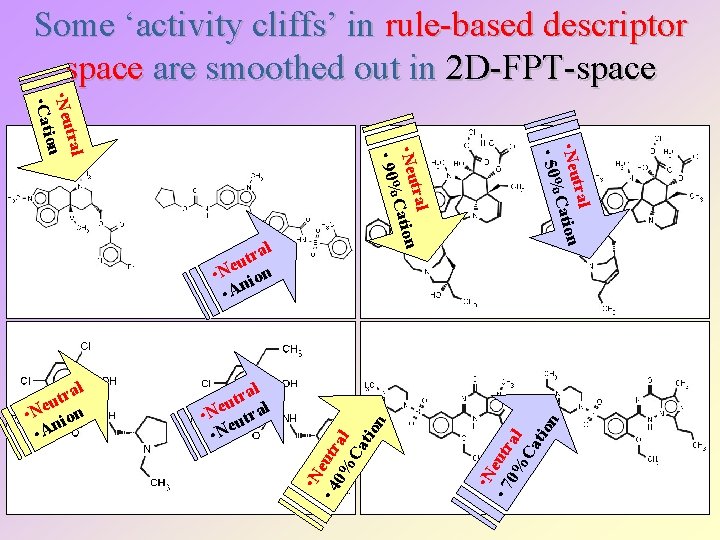
Some ‘activity cliffs’ in rule-based descriptor space are smoothed out in 2 D-FPT-space • N • 7 eutr 0% al Ca tio n • N • 4 eutr 0% al Ca tio n al r t u l • Ne utra • Ne tral • Neu Cation • 50% al r t u • Ne ion • An tral • Neu Cation • 90% tral • Neu n io • Cat al r t u • Ne ion • An

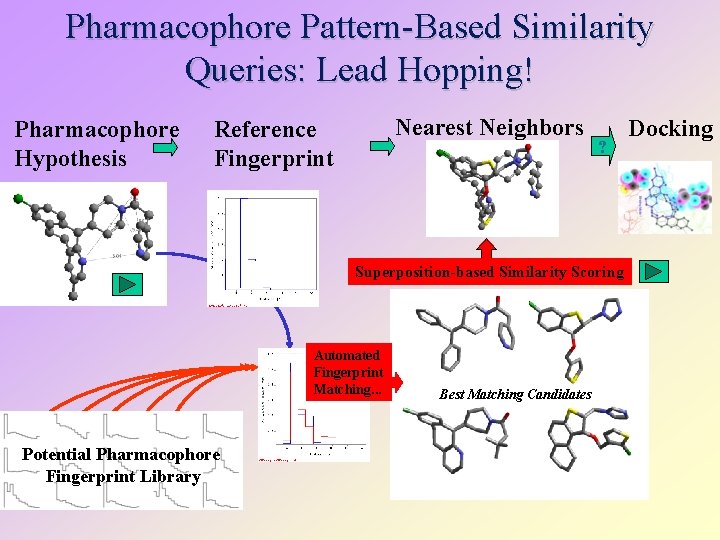
Pharmacophore Pattern-Based Similarity Queries: Lead Hopping! Pharmacophore Hypothesis Nearest Neighbors Reference Fingerprint ? Superposition-based Similarity Scoring Automated Fingerprint Matching. . . Potential Pharmacophore Fingerprint Library Best Matching Candidates Docking

Some examples of "hidden similarity"

Successful Virtual Screening Simulations D 2 TK

Successful QSAR model construction with 2 DFPT: predicting c-Met TK activity 25 variables entering nonlinear model 153 molecules for training: RMSE=0. 4 (log units), R 2=0. 82 40 molecules for validation: RMSE=0. 8 (log units), R 2=0. 53 8 validation molecules out of 40 mispredicted by more than 1 log

What more could be done? • 3 D FPT version under study – does it pay off to generate conformers? How many would you need to get better results than with 2 D-FPT? What’s the best conformational sampler to use? • Accessibility-weighted fingerprints? – class to return (topological and/or 3 D) estimate of the solventaccessible fraction of an atom? • Tautomer-dependent fingerprints? – if tautomers and their percentage were enumerated like any other microspecies…

THE END

Pharmacophore Hypotheses (A): From individual Active Leads: 2 D/3 D • ALL features in the Lead assumed relevant for binding (B): Consensus hypotheses from set of Leads: 2 D/3 D • Ignore features that can be deleted without losing activity (C): Site-Ligand interaction models: 3 D* • Select Ligand features shown to interact with the site in the 3 D X-ray structure of the site-ligand complex. (D): Active Site filling models: 3 D* • Design a pharmacophoric feature distribution complementary to the groups available in the active site * In these cases, docking may be performed starting from pharmacophore –based overlays


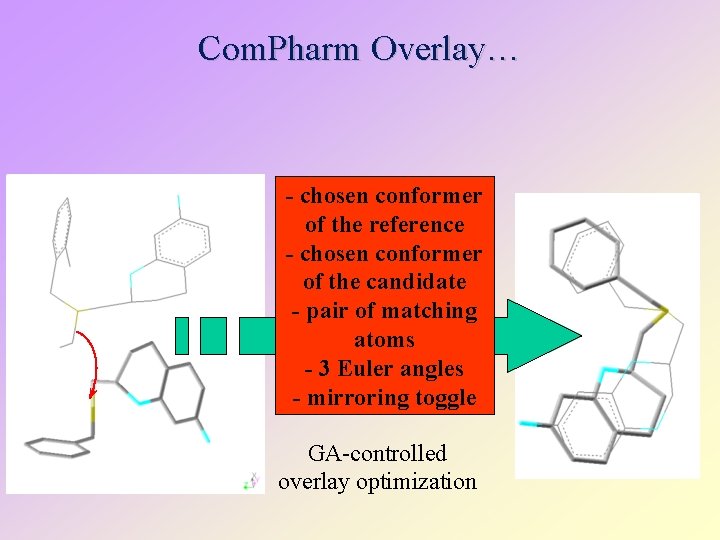
Com. Pharm Overlay… - chosen conformer of the reference - chosen conformer of the candidate - pair of matching atoms - 3 Euler angles - mirroring toggle GA-controlled overlay optimization

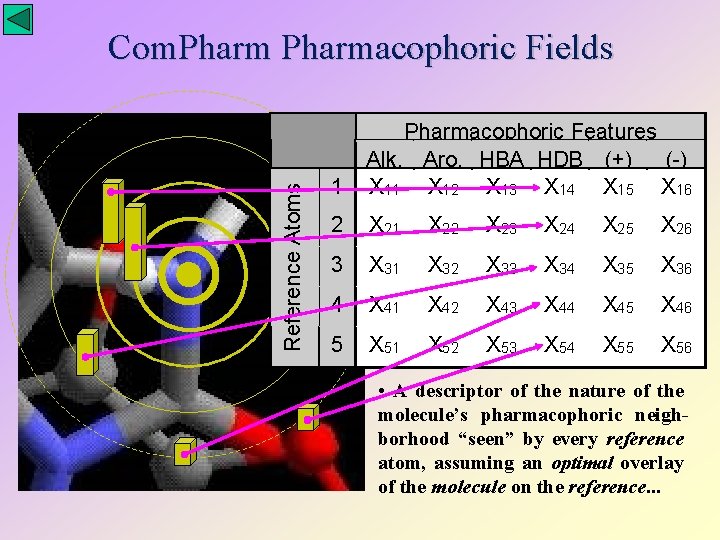
Reference Atoms Com. Pharmacophoric Fields 1 Pharmacophoric Features Alk. Aro. HBA HDB (+) (-) X 11 X 12 X 13 X 14 X 15 X 16 2 X 21 X 22 X 23 X 24 X 25 X 26 3 X 31 X 32 X 33 X 34 X 35 X 36 4 X 41 X 42 X 43 X 44 X 45 X 46 5 X 51 X 52 X 53 X 54 X 55 X 56 • A descriptor of the nature of the molecule’s pharmacophoric neighborhood “seen” by every reference atom, assuming an optimal overlay of the molecule on the reference. . .

 Lipinski rule of 5
Lipinski rule of 5 Three fundamental principles of fingerprints
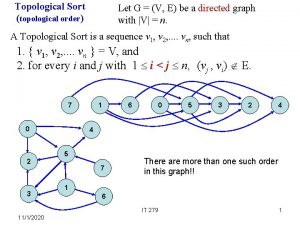
Three fundamental principles of fingerprints Topological sort bfs
Topological sort bfs Boundary descriptors in digital image processing
Boundary descriptors in digital image processing Topological sorting
Topological sorting Difference between insertion sort and bubble sort
Difference between insertion sort and bubble sort Dfs algorithm
Dfs algorithm Topological sort algorithm
Topological sort algorithm Topological sort calculator
Topological sort calculator Topological sort time complexity
Topological sort time complexity White path theorem
White path theorem Strongly connected components
Strongly connected components Search graph
Search graph Topological sort
Topological sort Topological sort calculator
Topological sort calculator Topological mott insulator
Topological mott insulator Topological band theory
Topological band theory Topological sort kahn's algorithm
Topological sort kahn's algorithm Graph topological sort
Graph topological sort Topological sort
Topological sort How to shrink a rubber band
How to shrink a rubber band Partial order relation
Partial order relation Topological sort online
Topological sort online Define topological sort in data structure
Define topological sort in data structure Kahn's algorithm python
Kahn's algorithm python Closed patterns and max-patterns
Closed patterns and max-patterns Obj dating
Obj dating Ridge enclosure
Ridge enclosure Chapter 6 fingerprints
Chapter 6 fingerprints Types of fingerprints
Types of fingerprints