Pharmacology of Antiviral Agents Tufts University School of







- Slides: 38

Pharmacology of Antiviral Agents Tufts University School of Medicine December 2016 Linden Hu, MD 1

TREATABLE VIRAL INFECTIONS • Influenza • Herpes viruses Herpes simplex Cytomegalovirus Varicella zoster virus • HIV • Hepatitis C • Hepatitis B 2

Mechanism of Action of Antiviral Agents • Inhibit one or more of the following: – Viral nucleic acid synthesis – Viral uncoating – Binding of viral particle to host cell Antiviral drugs must distinguish virus from host! 3

Influenza • Hemagglutinin – Site by which virus binds to host cell receptor • Neuraminidase – Degrades host cell receptor, allowing release of virus after replication has occurred 4

Approaches to Control of Influenza • Vaccination at start of influenza season (immunoprophylaxis) • Treatment with antiviral agents – Greatest effect when rx begun w/in 24 hrs 5

Antiviral Agents for Influenza M 2 Inhibitors (influenza A only) • Amantadine • Rimantadine Neuraminidase inhibitors • Oseltamavir (Tamiflu) • Zanamivir (Relenza) • Peramivir (Rapivab) 6

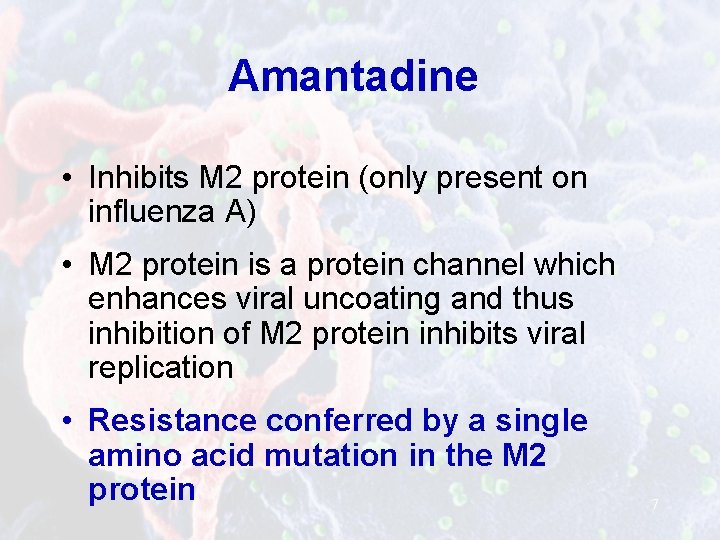
Amantadine • Inhibits M 2 protein (only present on influenza A) • M 2 protein is a protein channel which enhances viral uncoating and thus inhibition of M 2 protein inhibits viral replication • Resistance conferred by a single amino acid mutation in the M 2 protein 7

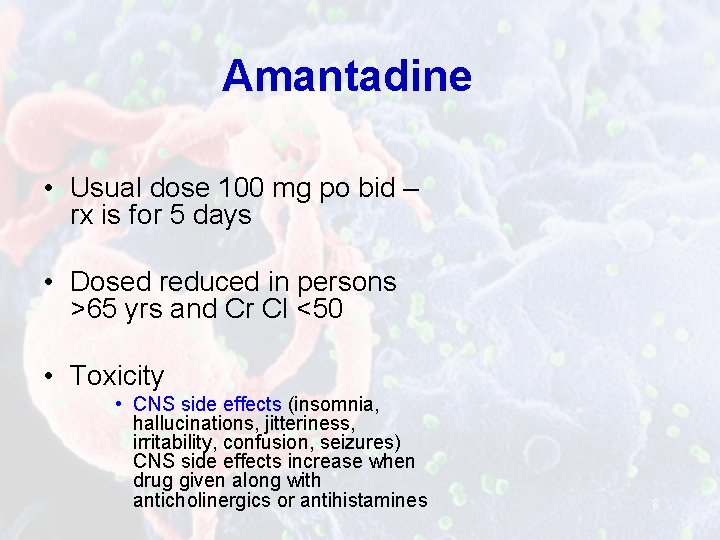
Amantadine • Usual dose 100 mg po bid – rx is for 5 days • Dosed reduced in persons >65 yrs and Cr Cl <50 • Toxicity • CNS side effects (insomnia, hallucinations, jitteriness, irritability, confusion, seizures) CNS side effects increase when drug given along with anticholinergics or antihistamines 8

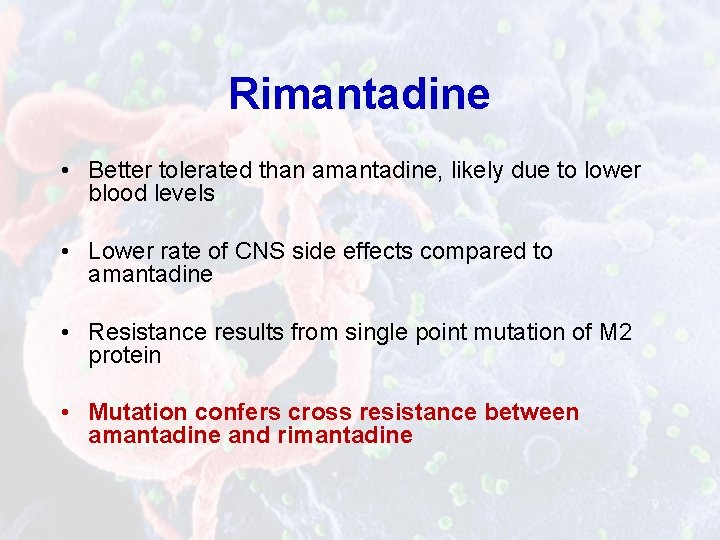
Rimantadine • Better tolerated than amantadine, likely due to lower blood levels • Lower rate of CNS side effects compared to amantadine • Resistance results from single point mutation of M 2 protein • Mutation confers cross resistance between amantadine and rimantadine 9

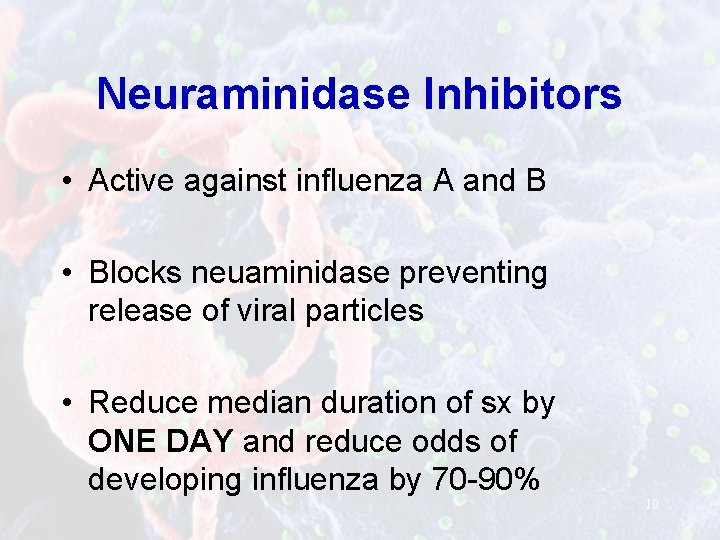
Neuraminidase Inhibitors • Active against influenza A and B • Blocks neuaminidase preventing release of viral particles • Reduce median duration of sx by ONE DAY and reduce odds of developing influenza by 70 -90% 10

Oseltamivir (Tamiflu) • Given orally – rapidly absorbed from GI tract • Excreted by kidney • Standard dose is 75 mg po bid • Can be given for treatment and prophylaxis • Main side effect is GI upset • Few reports of neurotoxicity in children 11

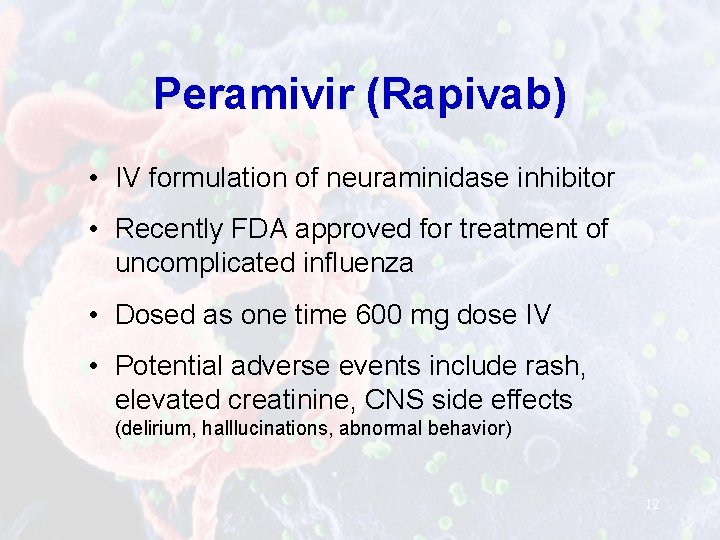
Peramivir (Rapivab) • IV formulation of neuraminidase inhibitor • Recently FDA approved for treatment of uncomplicated influenza • Dosed as one time 600 mg dose IV • Potential adverse events include rash, elevated creatinine, CNS side effects (delirium, halllucinations, abnormal behavior) 12

Zanamivir (Relenza) • Inhaled - not orally bioavailable • May precipitate bronchospasm • Decreases sx of influenza by 1. 5 days if given w/i 48 hrs of sx onset – also reduces severity of sx • Not widely used due to lack of oral administration • IV zanamivir is being evaluated in clinical trials and is available for compassionate use 13

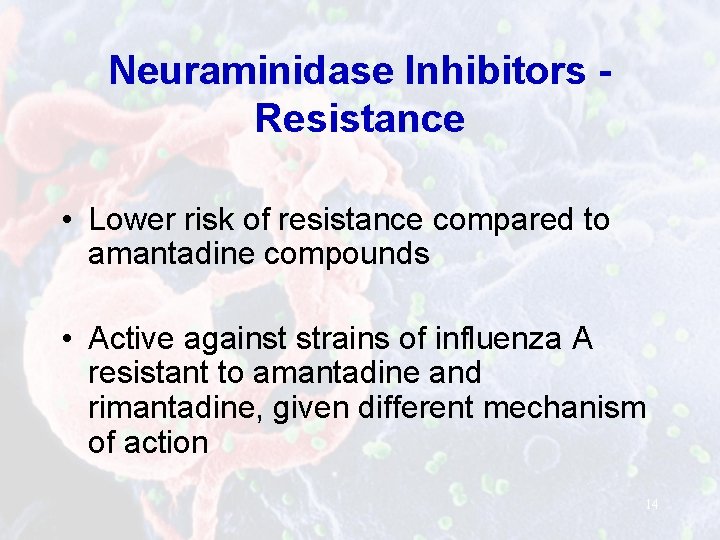
Neuraminidase Inhibitors Resistance • Lower risk of resistance compared to amantadine compounds • Active against strains of influenza A resistant to amantadine and rimantadine, given different mechanism of action 14

Indications for Antiviral Treatment for Suspected or Confirmed Influenza • Severe illness requiring hospitalization • Pregnancy • Age <2 years or >65 years • Chronic medical condition 15

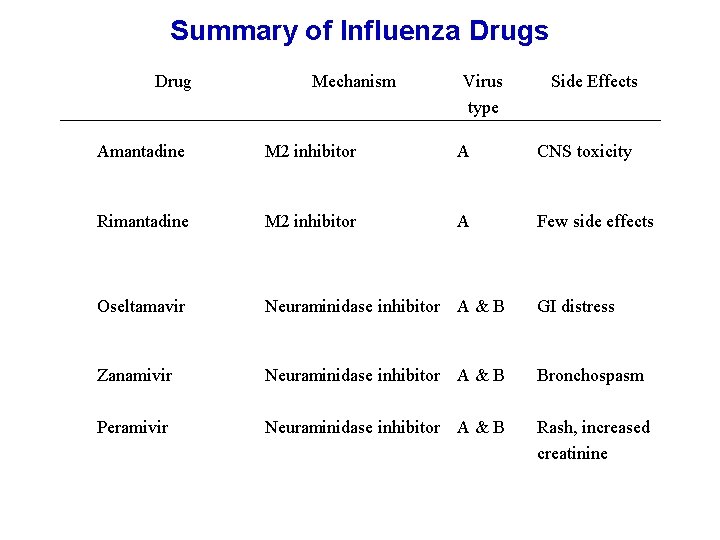
Summary of Influenza Drugs Drug Mechanism Virus type Side Effects Amantadine M 2 inhibitor A CNS toxicity Rimantadine M 2 inhibitor A Few side effects Oseltamavir Neuraminidase inhibitor A & B GI distress Zanamivir Neuraminidase inhibitor A & B Bronchospasm Peramivir Neuraminidase inhibitor A & B Rash, increased creatinine 16

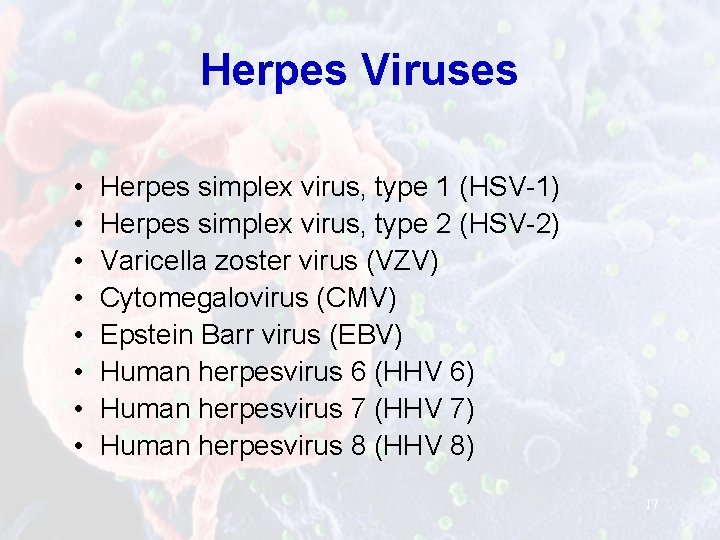
Herpes Viruses • • Herpes simplex virus, type 1 (HSV-1) Herpes simplex virus, type 2 (HSV-2) Varicella zoster virus (VZV) Cytomegalovirus (CMV) Epstein Barr virus (EBV) Human herpesvirus 6 (HHV 6) Human herpesvirus 7 (HHV 7) Human herpesvirus 8 (HHV 8) 17

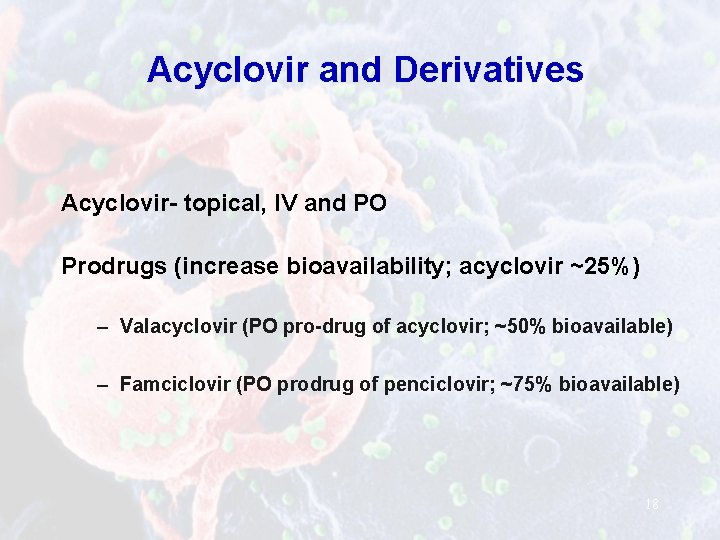
Acyclovir and Derivatives Acyclovir- topical, IV and PO Prodrugs (increase bioavailability; acyclovir ~25%) – Valacyclovir (PO pro-drug of acyclovir; ~50% bioavailable) – Famciclovir (PO prodrug of penciclovir; ~75% bioavailable) 18

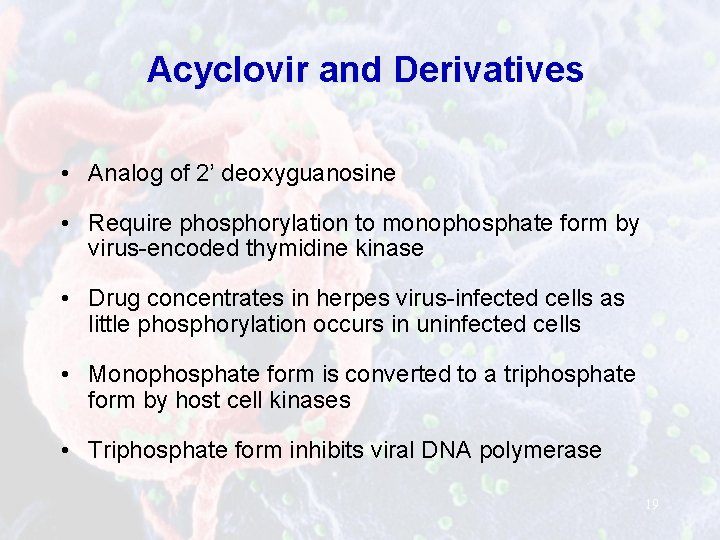
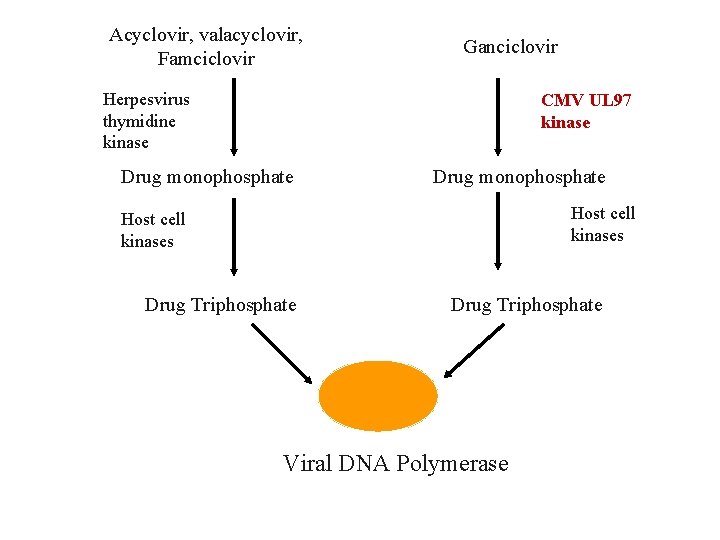
Acyclovir and Derivatives • Analog of 2’ deoxyguanosine • Require phosphorylation to monophosphate form by virus-encoded thymidine kinase • Drug concentrates in herpes virus-infected cells as little phosphorylation occurs in uninfected cells • Monophosphate form is converted to a triphosphate form by host cell kinases • Triphosphate form inhibits viral DNA polymerase 19

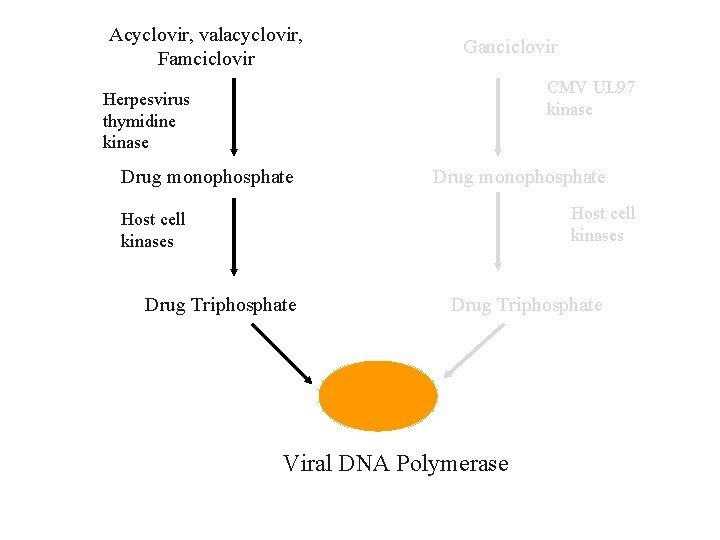
Acyclovir, valacyclovir, Famciclovir Ganciclovir CMV UL 97 kinase Herpesvirus thymidine kinase Drug monophosphate Host cell kinases Drug Triphosphate Viral DNA Polymerase 20

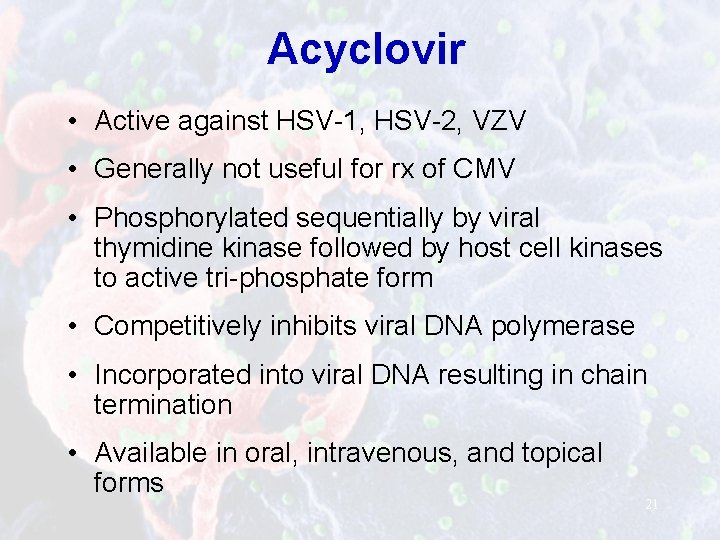
Acyclovir • Active against HSV-1, HSV-2, VZV • Generally not useful for rx of CMV • Phosphorylated sequentially by viral thymidine kinase followed by host cell kinases to active tri-phosphate form • Competitively inhibits viral DNA polymerase • Incorporated into viral DNA resulting in chain termination • Available in oral, intravenous, and topical forms 21

Clinical Use of Acyclovir HSV infections Genital HSV (primary, recurrent, suppression) Oral-Labial HSV Encephalitis Neonatal HSV Prophylaxis (prevent reactivation of HSV in immunocompromised hosts) VZV Varicella (chickenpox) Herpes zoster (shingles) 22

Acyclovir • Preparations – Topical – Oral (200, 800 mg) – Intravenous • Oral bioavailability 20 -30% • Penetrates well into CSF - CSF level 50% of serum level • >60% excreted unchanged in the urine 23

Acyclovir Toxicity • Nephrotoxicity (particularly after rapid intravenous infusion and inadequate hydration) • CNS – Agitation, lethargy, tremors – Hallucinations – Disorientation 24

Mechanisms of Resistance • Thymidine kinase deficient mutant • Thymidine kinase with altered substrate and unable to phosphorylate acyclovir • Mutations in viral DNA polymerase *Almost all clinically significant acyclovir resistance seen in immunocompromised hosts *Commonly associated with cross resistance to famciclovir and valacyclovir 25

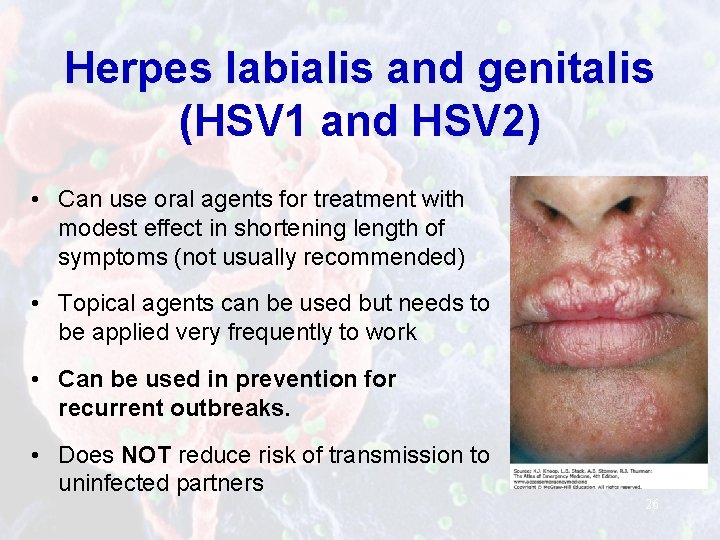
Herpes labialis and genitalis (HSV 1 and HSV 2) • Can use oral agents for treatment with modest effect in shortening length of symptoms (not usually recommended) • Topical agents can be used but needs to be applied very frequently to work • Can be used in prevention for recurrent outbreaks. • Does NOT reduce risk of transmission to uninfected partners 26

Chickenpox • Treat with acyclovir (or derivatives) • Treat immunocompromised hosts with varicella • Treat pregnant women in 2 nd and 3 rd trimester given risk of disseminated disease • Treat perinatal varicella • Treat adults >18 yrs; reduces number of lesions and accelerates time to crusting 27

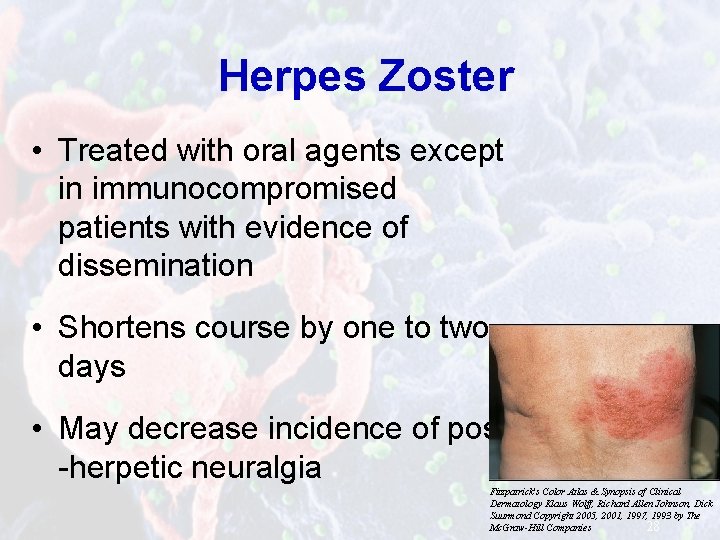
Herpes Zoster • Treated with oral agents except in immunocompromised patients with evidence of dissemination • Shortens course by one to two days • May decrease incidence of post -herpetic neuralgia Fitzpatrick's Color Atlas & Synopsis of Clinical Dermatology Klaus Wolff, Richard Allen Johnson, Dick Suurmond Copyright 2005, 2001, 1997, 1993 by The Mc. Graw-Hill Companies 28

Special Uses for Intravenous Acyclovir • Neonatal Herpes • HSV Encephalitis • Immunocompromised host with chickenpox or herpes zoster

Ganciclovir • Active against CMV, HSV-1, HSV-2 • Not commonly used to rx HSV given that it is more toxic than acyclovir 30

Ganciclovir • Guanosine derivative • Ganciclovir triphosphate selectively inhibits CMV DNA polymerase • Resistance common among persons treated for >3 months and is usually due to mutations in CMV UL 97 gene • Available in oral and intravenous forms 31

Acyclovir, valacyclovir, Famciclovir Ganciclovir Herpesvirus thymidine kinase CMV UL 97 kinase Drug monophosphate Host cell kinases Drug Triphosphate Viral DNA Polymerase 32

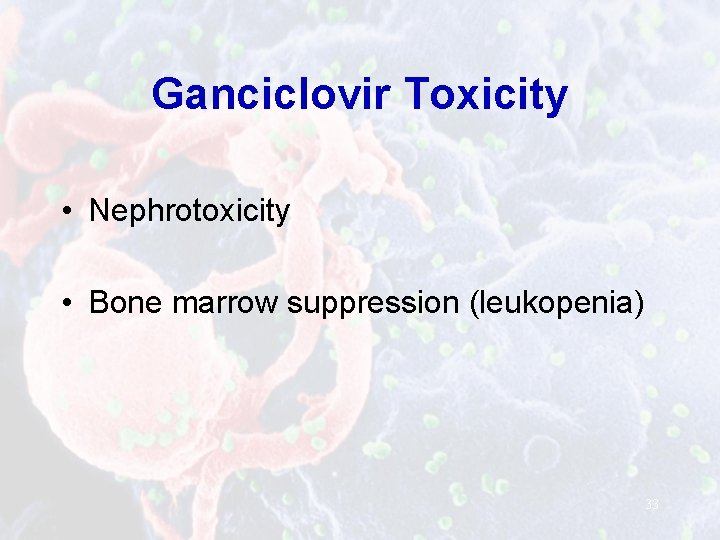
Ganciclovir Toxicity • Nephrotoxicity • Bone marrow suppression (leukopenia) 33

Valganciclovir • Orally bioavailable prodrug that is rapidly metabolized to ganciclovir in the intestine and liver • 60% of oral valganciclovir dose is absorbed • Used for prophylaxis of CMV disease in immunocompromised hosts and maintenance rx • IV ganciclovir used to treat severe CMV disease 34

Cidofovir • Nucleotide analogue that inhibits viral DNA polymerase • Activity against CMV, smallpox, BK virus, acyclovir resistant HSV • Long intracellular half-life that allows intermittent intravenous administration • Nephrotoxic 35

Foscarnet • Inhibits DNA polymerase – does not require phosphorylation • Effective against resistant CMV and HSV • Less well tolerated than ganciclovir and associated with considerable toxicity – Nephrotoxicity – Hypomagnesemia, hypokalemia, hypocalcaemia 36

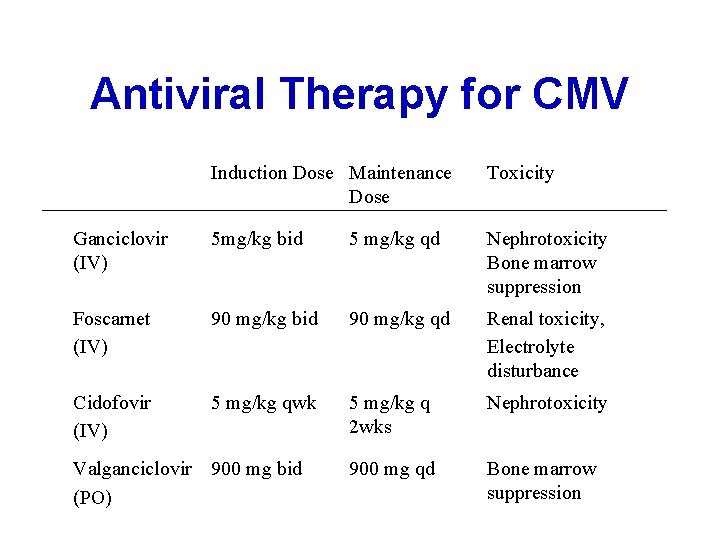
Antiviral Therapy for CMV Induction Dose Maintenance Dose Toxicity Ganciclovir (IV) 5 mg/kg bid 5 mg/kg qd Nephrotoxicity Bone marrow suppression Foscarnet (IV) 90 mg/kg bid 90 mg/kg qd Renal toxicity, Electrolyte disturbance Cidofovir (IV) 5 mg/kg qwk 5 mg/kg q 2 wks Nephrotoxicity 900 mg qd Bone marrow suppression Valganciclovir 900 mg bid (PO) 37

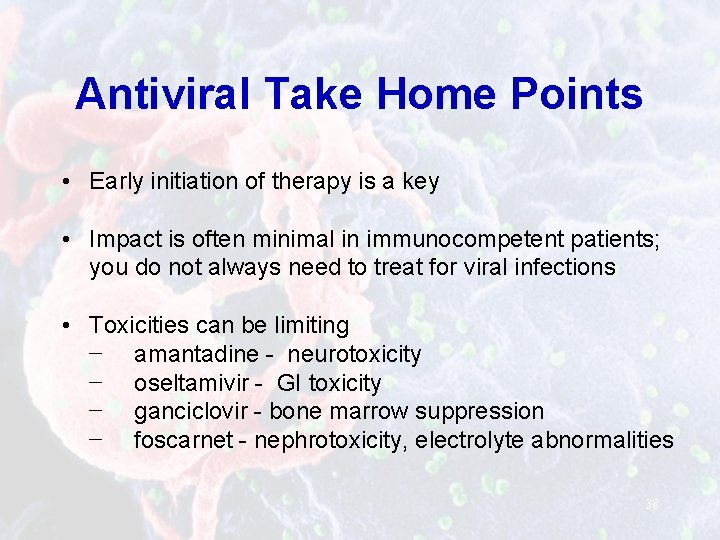
Antiviral Take Home Points • Early initiation of therapy is a key • Impact is often minimal in immunocompetent patients; you do not always need to treat for viral infections • Toxicities can be limiting − amantadine - neurotoxicity − oseltamivir - GI toxicity − ganciclovir - bone marrow suppression − foscarnet - nephrotoxicity, electrolyte abnormalities 38
 Second antiviral pill
Second antiviral pill Antiviral ajan
Antiviral ajan Tufts pre matriculation credits
Tufts pre matriculation credits Tufts hpc cluster
Tufts hpc cluster Tufts anesthesiology residents
Tufts anesthesiology residents Tufts ilvs
Tufts ilvs Tufts engineers without borders
Tufts engineers without borders Tufts science and technology center
Tufts science and technology center Ex college tufts
Ex college tufts Tufts tusk
Tufts tusk Tufts navigator gym reimbursement
Tufts navigator gym reimbursement Enamel spindle
Enamel spindle Retzius lines
Retzius lines Svm
Svm Pharmacology and venipuncture
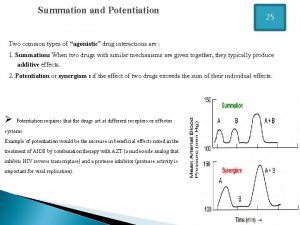
Pharmacology and venipuncture Drug summation examples
Drug summation examples Glomerular filtrate
Glomerular filtrate What is ion trapping in pharmacology
What is ion trapping in pharmacology Factors affecting drug metabolism
Factors affecting drug metabolism Chapter 30 principles of pharmacology
Chapter 30 principles of pharmacology First bypass effect
First bypass effect Alia drug testing
Alia drug testing Define pharmacology
Define pharmacology Factors affecting absorption of drug
Factors affecting absorption of drug Receptors in pharmacology
Receptors in pharmacology First pass metabolism definition pharmacology
First pass metabolism definition pharmacology Npte pharmacology
Npte pharmacology What is pharmacology
What is pharmacology Pharmacology introduction
Pharmacology introduction Competitive and non competitive antagonist
Competitive and non competitive antagonist What is ion trapping in pharmacology
What is ion trapping in pharmacology What is pharmacology
What is pharmacology Chapter 15 diagnostic procedures and pharmacology
Chapter 15 diagnostic procedures and pharmacology Pharmacology for nurses: a pathophysiological approach
Pharmacology for nurses: a pathophysiological approach Respiratory pharmacology quiz
Respiratory pharmacology quiz Pharmacology module
Pharmacology module Hepatic extraction ratio
Hepatic extraction ratio Clinical pharmacology powered by clinicalkey
Clinical pharmacology powered by clinicalkey Pharmacology of drugs acting on respiratory system
Pharmacology of drugs acting on respiratory system