Pharmacokinetics Chapters 8 and 11 1 Pharmacokinetics and







![Drug absorption -Henderson-Hasselbalch equation p. H= p. Ka + log [A-]/[HA] C-Size : Large Drug absorption -Henderson-Hasselbalch equation p. H= p. Ka + log [A-]/[HA] C-Size : Large](https://slidetodoc.com/presentation_image/43640ffa0c194049a7ba7cef8965692d/image-9.jpg)

























- Slides: 43

Pharmacokinetics Chapters 8 and 11 1

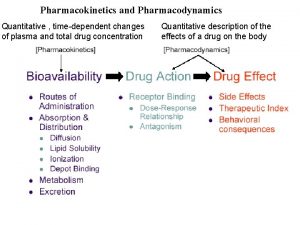
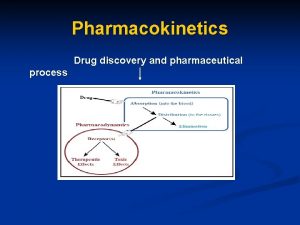
Pharmacokinetics and pharmacodynamics • Pharmacodynamics is the study of how drugs interact with a molecular target, i. e; effect of the drug on the body. • Pharmacokinetics is the study of how a drug reaches its target in the body and how it is affected on that journey, i. e; effect of the body on the drug. • Pharmacokinetics is the study of how is the drug absorbed, distributed, metabolized and excreted in the body 2

Pharmacokinetics & related topics The four main issues in Pharmacokinetics are: absorption, distribution, metabolism and excretion 3

Pharmacokinetics & related topics Drug absorption • Refers to the route or method by which the drug reaches the blood supply, this depends on how the drug is administered. • The most common and preferred method of administration is the oral route. • It depends on hydrophilic/hydrophobic properties, polarity and ionization of the drug. 4

Drug absorption I-Stability: ”Oral drugs have to be chemically stable to survive the stomach HCl and metabolically stable to survive the digestive enzymes in GIT and metabolic enzymes in liver (mainly cytochrome P 450 ). -Insulin, local anaesthetics and first penicillins are acid labile , so they can't be taken orally but are given parentrally. 5

Pharmacokinetics & related topics Drug absorption • II-Solubility • The drug should have the correct balance of water versus fat solubility • Oral drugs should be sufficiently polar to dissolve in the GIT and blood supply, but sufficiently fatty to pass through the cell membranes (optimum hydrophophobic/hydrophilic balance). • Most oral drugs obey Lipinski’s rule of five, i. e. 1 -A molecular weight less than 500 2 -No more than 5 hydrogen bond donor groups 3 -No more than 10 hydrogen bond acceptor groups 4 -A calculated log P value less than + 5 6

Pharmacokinetics & related topics Drug absorption • II-Solubility A- Polarity: • Some polar drugs break these rules are usually poorly absorbed and have to be administered by injection. • Highly polar drugs will dissolve in GIT but they will fail to be absorbed through the lipid cell membrane of the gut wall while nonpolar drugs will be poorly soluble in the GIT instead they will dissolve in the fat globules leading to poor surface contact with cell membranes resulting in poor absorption , too. 7

Pharmacokinetics & related topics Drug absorption B-Ionization: The presence of the weak ionizable -NH- group in many drug structure would have three advantages: A- good solubility due to =NH 2+ cation in stomach acid B- good absorption due to conversion to non ionized form in intestine in slightly alkaline p. H C-good target interactions due to participation of ammonium ion in them 8
![Drug absorption HendersonHasselbalch equation p H p Ka log AHA CSize Large Drug absorption -Henderson-Hasselbalch equation p. H= p. Ka + log [A-]/[HA] C-Size : Large](https://slidetodoc.com/presentation_image/43640ffa0c194049a7ba7cef8965692d/image-9.jpg)
Drug absorption -Henderson-Hasselbalch equation p. H= p. Ka + log [A-]/[HA] C-Size : Large molecular weight drugs generally have poor absorption because they mostly have a large number of polar groups which will lead to poor absorption of these drugs. 9

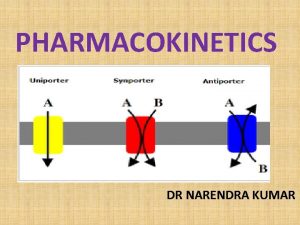
Pharmacokinetics & related topics Drug absorption mechanisms: • Most drugs with proper solubility in both water and lipid will be absorbed through the lipid cell membrane of the gut wall cells. • Carrier proteins are essential to a cell’s survival as they transport highly polar building blocks required for various biosynthetic pathways. 10

Pharmacokinetics & related topics Drug absorption mechanisms: • Some polar drugs, are absorbed by special carrier proteins such as levodopa fluorouracil, lisinopril, methotrexate and erythromycin, which are similar in structure to (or bear a structural resemblance to) one of the building blocks (such as amino acid) then it too may be smuggled into the cell • Other polar drugs with high molecular weight are absorbed by pinocytosis (without passing through the membrane). 11

Pharmacokinetics & related topics Drug absorption mechanisms: • Some polar drugs with low molecular weight (<200) are absorbed by passing through the pores between cells lining the gut wall. • Thus polar drugs are orally active if they are small enough to pass between the cells of the gut wall or are recognized by carrier proteins or are taken across the gut wall by pinocytosis. • N. B: sometimes drugs are designed to be highly polar to be retained in the gut and not absorbed to treat gut infections as some antibacterial agents for gut infections. 12

Pharmacokinetics & related topics • Dosage: • Drug dosing or dose regimen (regime) means drug amount in each dose and frequency of administration. • Due to the number of pharmacokinetic variables involved, it can be difficult to estimate the correct dosing regime for a drug. • The drug should be administered at the correct dose levels and at frequency to ensure that blood concentration remain within therapeutic window. • Therapeutic window means drug levels in blood lie between therapeutic and toxic levels). 13

Pharmacokinetics & related topics • Dosage: • In general, the concentration of free drug in blood (non bound to plasma proteins) is a good indication of the availability of that drug at its target site. • Other dosing complications include differences in age, sex, race, diet, environment, obesity, time of dosing (due to change in metabolic rates throughout the day), drug-drug interactions. 14

Dosage • The half-life of a drug is the time taken for the blood concentration of the drug to fall by half. A knowledge of half-life is required to calculate how frequently doses should be given to ensure a steady state concentration. • Drug tolerance is where the effect of a drug diminishes after repeated doses. • In physical dependence a patient becomes dependent on a drug and suffers withdrawal symptoms on stopping the treatment. 15

Formulation of drug § Formulation refers to the method by which the drugs are prepared for administration, where by solution, pill, capsule, liposome (small vesicles consisting of a phospholipid bilayer mambrane), or microsphere (small spheres made up of a biologically degradable polymer § The way a drug is formulated can avoid some of the problems associated with oral administration. § Drugs are normally taken orally as tablets or capsules. § A tablet is a compressed preparation that contains 510% of the drug (active ingredient), in addition to many additives which help to ensure easy disintegration, and dissolution of the tablet in the stomach or intestine. 16

Formulation of drug § Tablet formulation can be modified to give rapid effect (sublingual tablets) or sustained release. Special coatings can make the tablet resistant to stomach acid but disintegrates only in intestine (enteric coated tablets). Drug administration • The main routes are oral, sublingual, rectal, topical, epithelial, inhalation and injections. The method chosen depends upon the target organ and the pharmacokinetics of the drug. 17

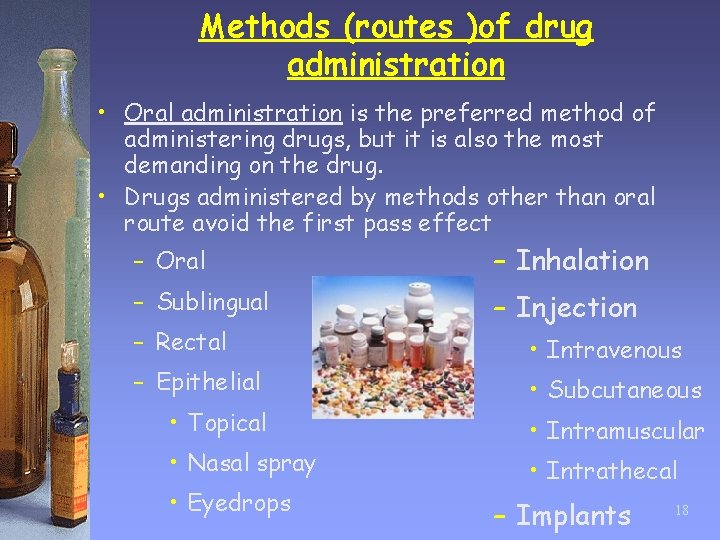
Methods (routes )of drug administration • Oral administration is the preferred method of administering drugs, but it is also the most demanding on the drug. • Drugs administered by methods other than oral route avoid the first pass effect – Oral – Inhalation – Sublingual – Injection – Rectal – Epithelial • Topical • Nasal spray • Eyedrops • Intravenous • Subcutaneous • Intramuscular • Intrathecal – Implants 18

Drug administration o Drugs can be administered such that they are absorbed through the mucous membranes of the mouth, nose, or eyes. o Some drugs are administered rectally as suppositories. o Topically administered drugs are applied to the skin. Some drugs are absorbed through the skin into the blood supply. o Inhaled drugs are administered as gases or aerosols to act directly on the respiratory system. Some inhaled drugs are absorbed into the blood supply to act systemically. 19

Drug administration o Polar drugs which are unable to cross cell membranes are given by injection. o Injection is the most efficient method of administering a drug but it also the most hazardous. Injection can be intravenous, intramuscular, subcutaneous, or intrathecal. o Implants have been useful in providing controlled drug release such that blood concentrations of the drug remain as level as possible. (e. g. insulin, gliadel) 20

Drug distribution • Once the drug is absorbed, it is rapidly distributed around the blood supply, then slowly distributed to the various tissues and organs. • Distribution to the interstitial fluid surrounding tissues and organs is rapid if the drug is not bound to plasma proteins. • Some drugs have to enter cells in order to reach their target. • A certain percentage of a drug may be absorbed into fatty tissue (e. g. Barbiturates) and/or bound to macromolecules 21

Drug distribution • Drugs entering the CNS have to cross the bloodbrain barrier. Polar drugs (e. g. Penicillin) are unable to cross this barrier unless they make use of carrier proteins or are taken across by pinocytosis (e. g. insulin). • Some drugs cross the placental barrier into the fetus and may harm development or prove toxic in newborn babies (e. g. alcohol, nicotine, cocaine, barbiturates) 22

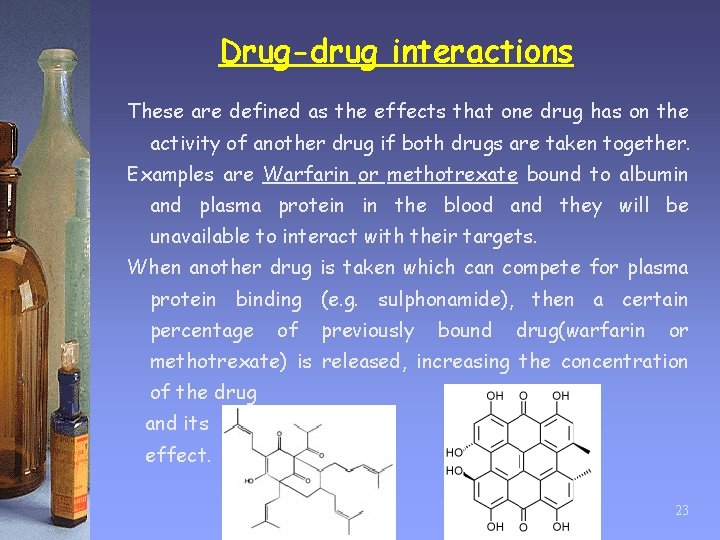
Drug-drug interactions These are defined as the effects that one drug has on the activity of another drug if both drugs are taken together. Examples are Warfarin or methotrexate bound to albumin and plasma protein in the blood and they will be unavailable to interact with their targets. When another drug is taken which can compete for plasma protein binding (e. g. sulphonamide), then a certain percentage of previously bound drug(warfarin or methotrexate) is released, increasing the concentration of the drug and its effect. 23

Drug Metabolism • Drugs are exposed to enzyme-catalyzed reactions which modify their structure. This is called drug metabolism and can take place in various tissues. But, most reactions occur in liver. • Orally taken drugs are subjected to the first pass effect. • Drugs administered by methods other than the oral route avoid the first pass effect. • Phase I metabolic reactions typically involve the addition or exposure of a polar functional group. 24

Drug Metabolism • Cytochrome P 450 enzymes present in the liver carry out important phase I oxidation reactions. The type of cytochrome P 450 enzymes present vary between individuals, leading to varying rates of metabolism. • The activity of cytochrome P 450 enzymes can be affected by food, chemicals, and drugs, resulting in drug-drug interactions and possible side effects. • Phase II metabolic reactions involve the addition of highly polar molecules to a functional group. The resulting conjugate are more easily excreted. 25

Drug Excretion • Drugs excretion can take place through sweat, exhaled air, or bile, but most excretion takes place through the kidneys. • The kidneys filter blood such that drugs and their metabolites enter nephrons. • Non-polar substances are reabsorbed into the blood supply, but polar substances are retained in the nephrons and excreted in urine. 26

To control chemical and physical properties… • Drug design • Alter functional groups • Quantitative SARs • Computational methods 27

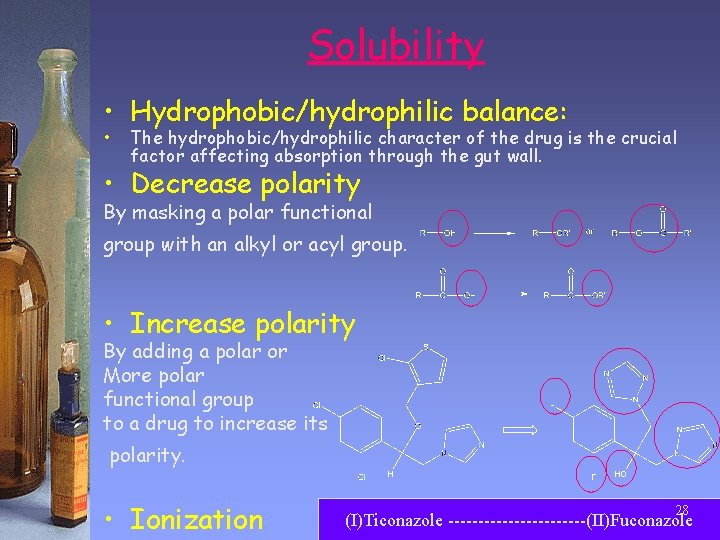
Solubility • Hydrophobic/hydrophilic balance: • The hydrophobic/hydrophilic character of the drug is the crucial factor affecting absorption through the gut wall. • Decrease polarity By masking a polar functional group with an alkyl or acyl group. • Increase polarity By adding a polar or More polar functional group to a drug to increase its polarity. • Ionization 28 (I)Ticonazole ------------(II)Fuconazole

• 1. • • • Stability Option A: Make drugs more resistant to metabolism and hydrolysis Steric shield Some functional groups are susceptible to chemical and enzymatic degradation than others. To protect such groups, a steric shield designed to hinder the approach of a nucleophile or enzyme to those groups is added. These usually involve the addition of a bulky alkyl group close to the functional group. E. g. , t-butyl group. 29

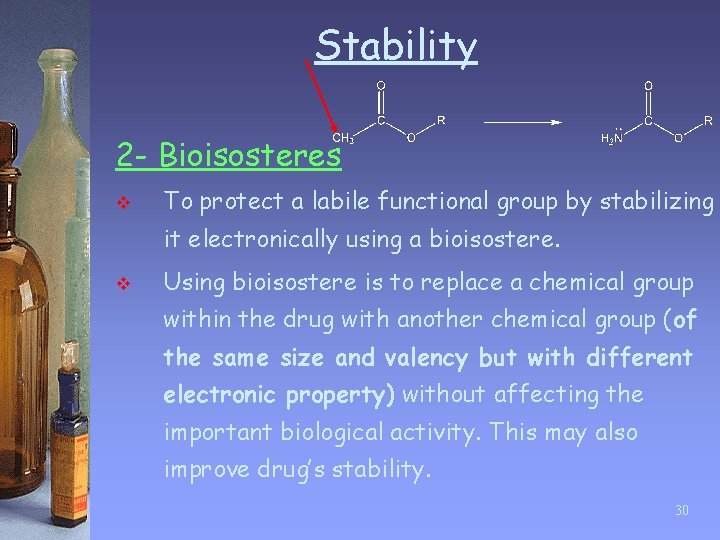
Stability 2 - Bioisosteres v To protect a labile functional group by stabilizing it electronically using a bioisostere. v Using bioisostere is to replace a chemical group within the drug with another chemical group (of the same size and valency but with different electronic property) without affecting the important biological activity. This may also improve drug’s stability. 30

Stability 3 - Stereo electronic modification ü ü This means steric hinderance together with electronic stabilization are used stabilize labile groups. Example: lidocaine from procaine Proocaine is short lasting due to quick hydrolysis of its ester group. Changing this ester group into less reactive amide reduces chemical hydrolysis. Moreover, the presence of two o-methyl groups on aromatic ring provides a steric shield for the carbonyl group. Lidocaine Proocaine or novocaine 31

Stability 4. Metabolic blockers v v v Example: Some drugs are metabolized by the introduction of polar groups at particular positions in their skeleton. Megestrol acetate is oxidized at position 6 to give a hydroxyl group, leading to quick elimination of the water soluble conjugate. By introducing a methyl group in its analogue at this position, metabolism is blocked and its action is prolonged. Megestrol acetate analogue 32

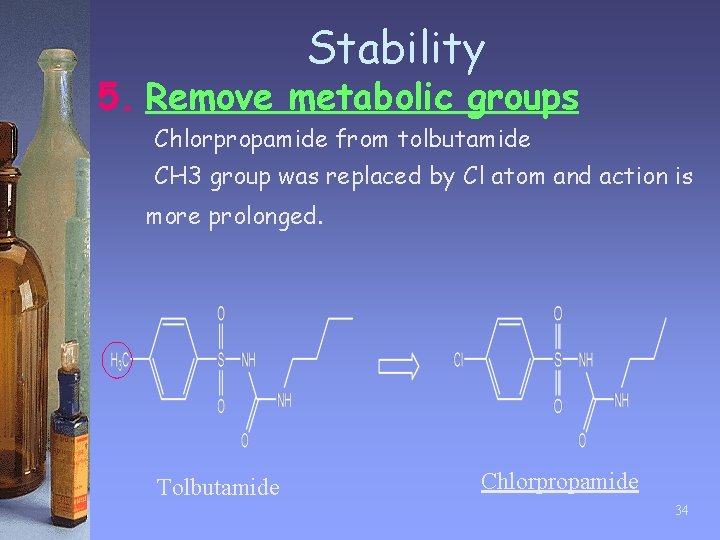
Stability 5. Remove metabolic groups • • • Certain chemical groups are susceptible to metabolic enzymes. Example: CH 3 -group on aromatic ring are often oxidized into –COOH, which can be quickly eliminated from the body. These groups are either removed or replaced by groups that are stable to oxidation to prolong the lifetime of the drug. 33

Stability 5. Remove metabolic groups Chlorpropamide from tolbutamide CH 3 group was replaced by Cl atom and action is more prolonged. Tolbutamide Chlorpropamide 34

Stability 6. Group Shifts When a vulnerable chemical group can’t be replaced or removed because it is involved in important binding interactions with the binding site, we have two possible solutions. One is to mask this group temporarily by using a prodrug. The second is to try shifting this group within the molecular skeleton. 35

Stability 6. Group Shifts Example: Salbutamol from noradrenaline, where in noradrenaline, both phenolic groups are involved in hydrogen bonding to the receptor, thus metabolic methylation of one of these groups makes the compound inactive and has a short duration. A solution is to move one phenolic group out from the ring by one carbon unit only (if more, activity is lost, due to improper binding) Salbutamol Noradrenaline 36

Stability • Option B: Make drugs less resistant to metabolism: • A drug that is extremely stable to metabolism and is very slowly excreted can pose just as many problems as one that is susceptible to metabolism. • If the effects of the drug could last too long then it would cause both: → Toxicity → Lingering side effects Therefore, designing drugs with decreased chemical and metabolic stability can sometimes be useful. This is called shortening the lifetime of the drug. 37

Other factors in drug design • Reducing Toxicity: • A drug fails clinical trials because of its toxic side effects. This may be due to toxic metabolites. • Thus, the drug should be made more resistant to metabolism by knowing functional groups prone to producing toxic metabolites and removing them or changing them into harmless substituents or varying their position. 38

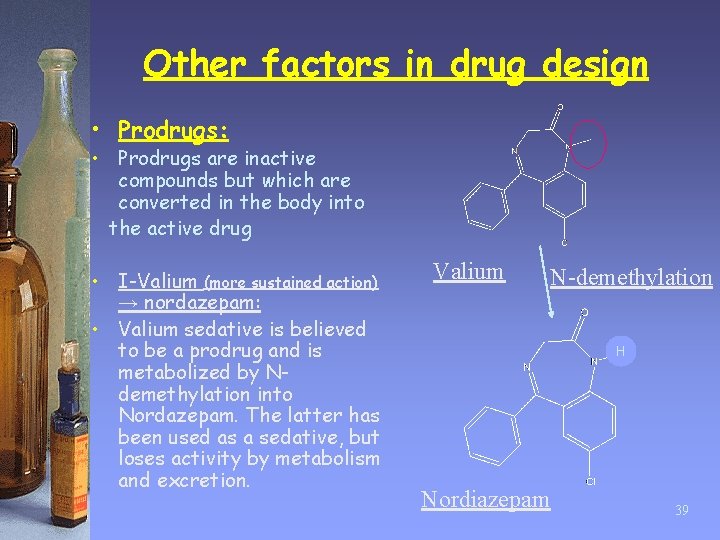
Other factors in drug design • Prodrugs: • Prodrugs are inactive compounds but which are converted in the body into the active drug • I-Valium (more sustained action) → nordazepam: • Valium sedative is believed to be a prodrug and is metabolized by Ndemethylation into Nordazepam. The latter has been used as a sedative, but loses activity by metabolism and excretion. Valium N-demethylation H Nordiazepam 39

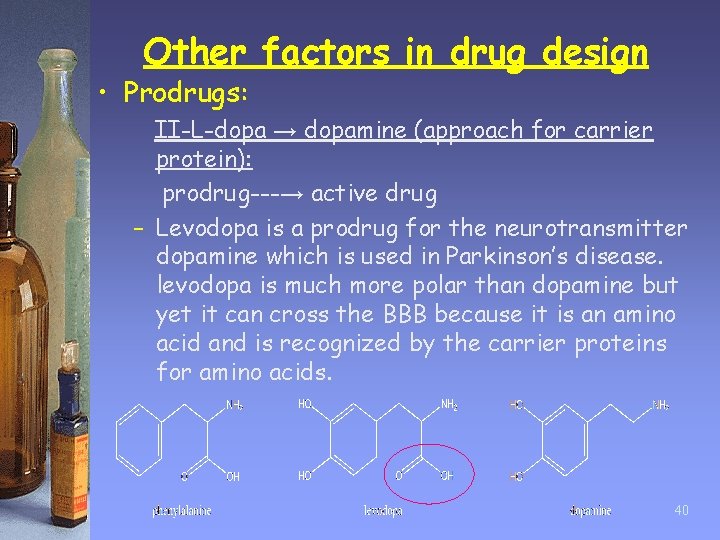
Other factors in drug design • Prodrugs: II-L-dopa → dopamine (approach for carrier protein): prodrug---→ active drug – Levodopa is a prodrug for the neurotransmitter dopamine which is used in Parkinson’s disease. levodopa is much more polar than dopamine but yet it can cross the BBB because it is an amino acid and is recognized by the carrier proteins for amino acids. 40

Other factors in drug design • Prodrugs: III-Aspirin → salicylic acid: To mask drug toxicity and side effects Salicylic acid is a good painkiller but causes gastric bleeding due to phenolic OH which is converted into an ester in aspirin. The ester is later hydrolysed to free the active drug. Also, aspirin is an antiinflammatory action. 41

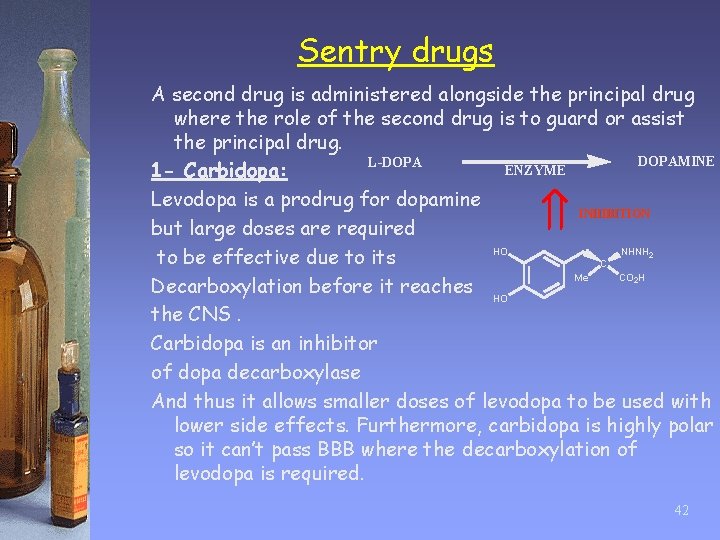
Sentry drugs A second drug is administered alongside the principal drug where the role of the second drug is to guard or assist the principal drug. DOPAMINE L-DOPA ENZYME 1 - Carbidopa: Levodopa is a prodrug for dopamine INHIBITION but large doses are required HO NHNH to be effective due to its C Me CO H Decarboxylation before it reaches HO the CNS. Carbidopa is an inhibitor of dopa decarboxylase And thus it allows smaller doses of levodopa to be used with lower side effects. Furthermore, carbidopa is highly polar so it can’t pass BBB where the decarboxylation of levodopa is required. 2 2 42

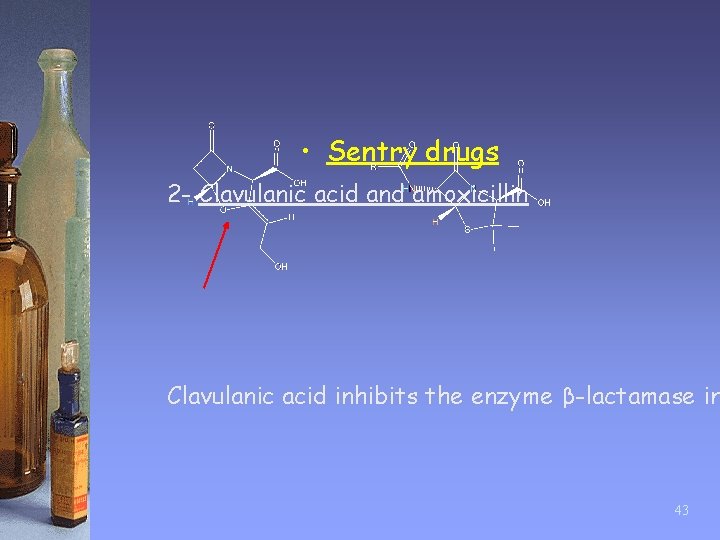
• Sentry drugs 2 - Clavulanic acid and amoxicillin Clavulanic acid inhibits the enzyme β-lactamase in 43
 Factors affecting drug metabolism slideshare
Factors affecting drug metabolism slideshare Pharmacokinetic definition
Pharmacokinetic definition First order elimination
First order elimination Drug metabolism and pharmacokinetics
Drug metabolism and pharmacokinetics Linear pharmacokinetics
Linear pharmacokinetics Define relative bioavailability
Define relative bioavailability Cmax in pharmacokinetics
Cmax in pharmacokinetics Pharmacokinetics vs pharmacodynamics
Pharmacokinetics vs pharmacodynamics Objectives of pharmacokinetics
Objectives of pharmacokinetics Nonlinear pharmacokinetics
Nonlinear pharmacokinetics Non linear pharmacokinetics
Non linear pharmacokinetics Non linear pharmacokinetics
Non linear pharmacokinetics Multi compartment model pharmacokinetics
Multi compartment model pharmacokinetics Nebido half life
Nebido half life Individualization of drug dosage regimen
Individualization of drug dosage regimen Alteplase pharmacokinetics
Alteplase pharmacokinetics Linear pharmacokinetics
Linear pharmacokinetics What is pharmacokinetics
What is pharmacokinetics Pharmacokinetics
Pharmacokinetics Pharmacokinetics
Pharmacokinetics To kill a mockingbird chapter 10-11 summary
To kill a mockingbird chapter 10-11 summary Chapter 1-3 summary to kill a mockingbird
Chapter 1-3 summary to kill a mockingbird Noughts and crosses chapter 8 summary
Noughts and crosses chapter 8 summary Chapter 4 summary
Chapter 4 summary Jekyll and hyde chapter 3
Jekyll and hyde chapter 3 Charlie and the chocolate factory chapter 14
Charlie and the chocolate factory chapter 14 Chapter 10 and 11 to kill a mockingbird
Chapter 10 and 11 to kill a mockingbird Allusion in the scarlet letter
Allusion in the scarlet letter Volume 3 chapter 10 pride and prejudice
Volume 3 chapter 10 pride and prejudice Lord of the flies chapter 9 symbols
Lord of the flies chapter 9 symbols The scarlet letter chapter 21-22 summary
The scarlet letter chapter 21-22 summary The watsons go to birmingham vocabulary
The watsons go to birmingham vocabulary How does morrie feel about music
How does morrie feel about music Catcher in the rye chapter 3 quotes
Catcher in the rye chapter 3 quotes Chapters 22-23 to kill a mockingbird
Chapters 22-23 to kill a mockingbird What was scouts first crime at school
What was scouts first crime at school Chapters 4-7 to kill a mockingbird summary
Chapters 4-7 to kill a mockingbird summary To kill a mockingbird chapter 4
To kill a mockingbird chapter 4 To kill a mockingbird 28-31 summary
To kill a mockingbird 28-31 summary To kill a mockingbird chapter 27-31 summary
To kill a mockingbird chapter 27-31 summary What is chapter 22 about in to kill a mockingbird
What is chapter 22 about in to kill a mockingbird To kill a mockingbird chapters 12-16
To kill a mockingbird chapters 12-16 Chapters 1-5 to kill a mockingbird summary
Chapters 1-5 to kill a mockingbird summary Fractious definition to kill a mockingbird
Fractious definition to kill a mockingbird