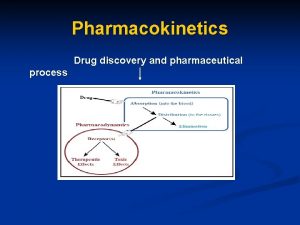
Working group on Pharmacokinetics and Population Pharmacokinetics of










- Slides: 12

Working group on Pharmacokinetics and Population Pharmacokinetics of factor concentrates Alfonso Iorio, on behalf of the working group

DISCLOSURES • Financial conflicts of interests – Mc. Master University has received funds for research and service agreements from Bayer, Baxalta, Biogen, Novo. Nordisk, Octapharma, and Pfizer. • Non-financial (intellectual) conflicts – PI for the WAPPS project (www. wapps-hemo. org) • No mention of unlicensed products

WORKING GROUP • Core members – A Iorio, V Blanchette, P Collins, K Fischer, J Blatny, E Neufeld • Additional members – D Lillicrap, D Hart, M Makris • PK and Pop. PK consultants – A Edginton, S Ito

Previous SSC Communications The design and analysis of half-life and recovery studies for factor VIII and factor IX. Factor VIII/Factor IX Scientific and Standardization Committee of the International Society for Thrombosis and Haemostasis. Morfini M, Lee M, Messori A. Thromb Haemost. 1991 Sep 2; 66(3): 384 -6.

Previous SSC Communications Scientific and Standardization Committee Communication The Design and Analysis of Pharmacokinetic Studies of Coagulation Factors On behalf of the Subcommittee on Factor VIII and Factor IX of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis M. Lee, M. Morfini, S. Schulman, J. Ingerslev and the Factor VIII/Factor IX Scientific and Standardization Committee of the ISTH Posted on the ISTH SSC on March 21, 2001

Previous SSC Communications … the approaches recommended here are primarily intended for clinical trials of factor concentrates that are being compared to other, similar preparations. … studies on individual patients for diagnostic or therapeutic purposes may not necessarily be relevant to what is discussed here.

Previous SSC Communications

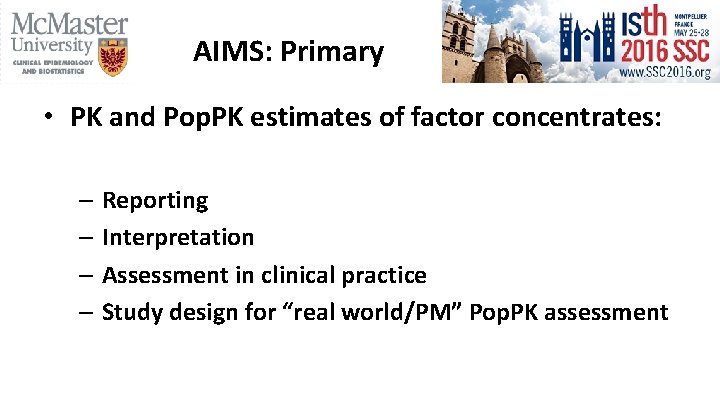
AIMS: Primary • PK and Pop. PK estimates of factor concentrates: – Reporting – Interpretation – Assessment in clinical practice – Study design for “real world/PM” Pop. PK assessment

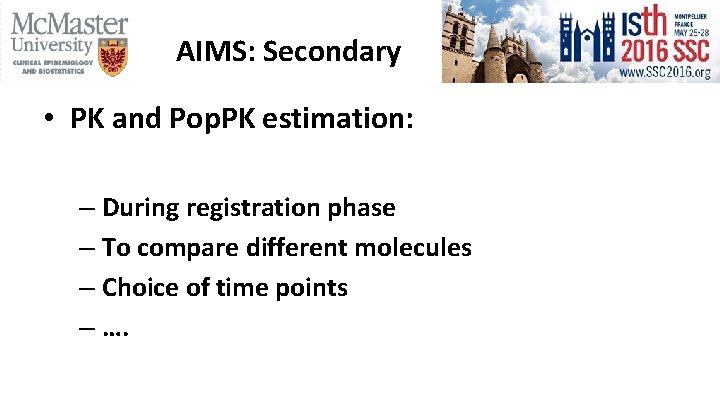
AIMS: Secondary • PK and Pop. PK estimation: – During registration phase – To compare different molecules – Choice of time points – ….

PROCESS • How (and whether) to best involve: – Pharmaceutical companies PK/Pop. PK experts – FDA / EMA PK/Pop. PK experts – Authors of PK/Pop. PK published papers – Patient representatives

PROGRESS • • • 2 conference calls 1 panel survey Bi-monthly conference calls Review of the existing literature Review of regulators guidance Draft recommendations on primary goals

Questions time Thank you for attention Slides posted on: http: //hemophilia. mcmaster. ca/resources
 Hot working and cold working difference
Hot working and cold working difference Hot working vs cold working
Hot working vs cold working Differentiate between hot working and cold working
Differentiate between hot working and cold working Contoh hot working
Contoh hot working Smart work and hard work
Smart work and hard work Chapter 4 population ecology section 1 population dynamics
Chapter 4 population ecology section 1 population dynamics Section 1 population dynamics answer key
Section 1 population dynamics answer key Population ecology section 1 population dynamics
Population ecology section 1 population dynamics Chapter 4 population ecology answer key
Chapter 4 population ecology answer key Difference between pharmacodynamics and pharmacokinetics
Difference between pharmacodynamics and pharmacokinetics Difference between pharmacokinetics and pharmacodynamics
Difference between pharmacokinetics and pharmacodynamics Introduction to biopharmaceutics and pharmacokinetics
Introduction to biopharmaceutics and pharmacokinetics Drug metabolism and pharmacokinetics
Drug metabolism and pharmacokinetics