Paulings Rules GLY 4200 Fall 2019 D L





- Slides: 19

Pauling’s Rules GLY 4200 Fall, 2019 © D. L. Warburton 2019 1

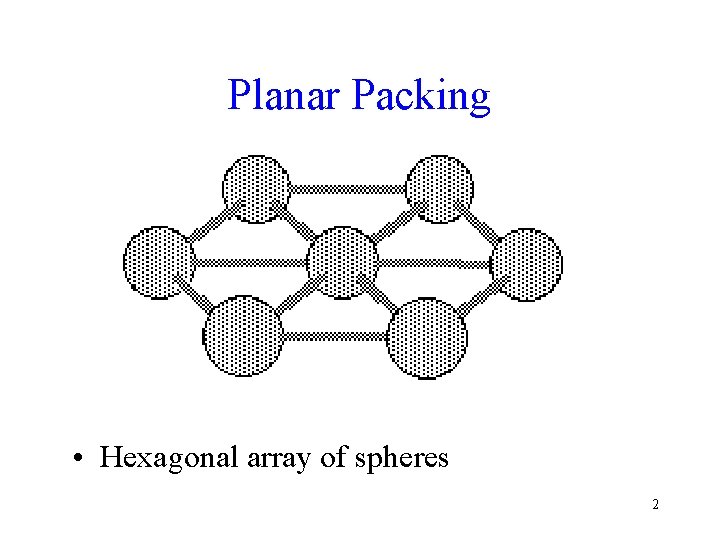
Planar Packing • Hexagonal array of spheres 2

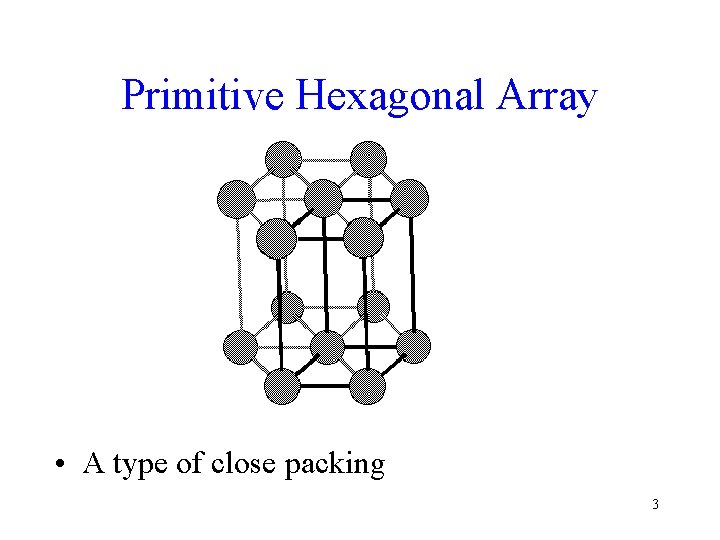
Primitive Hexagonal Array • A type of close packing 3

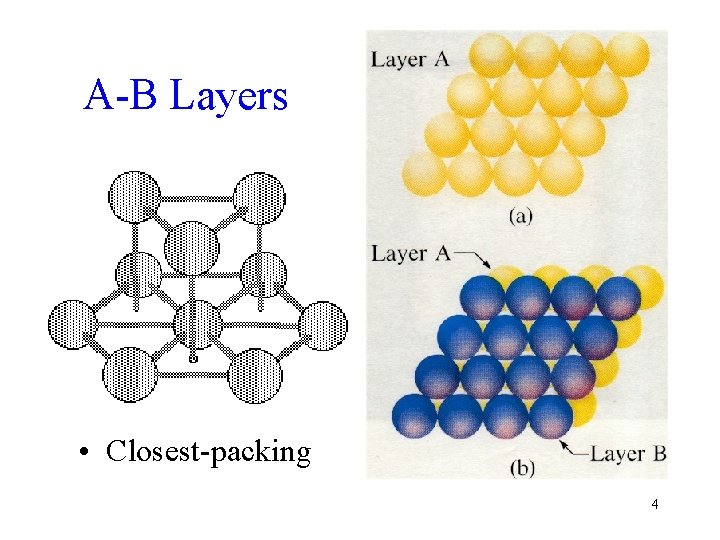
A-B Layers • Closest-packing 4

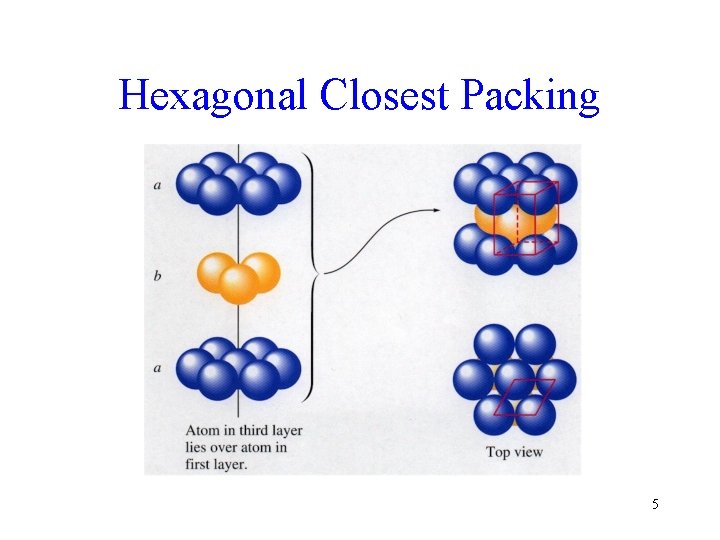
Hexagonal Closest Packing 5

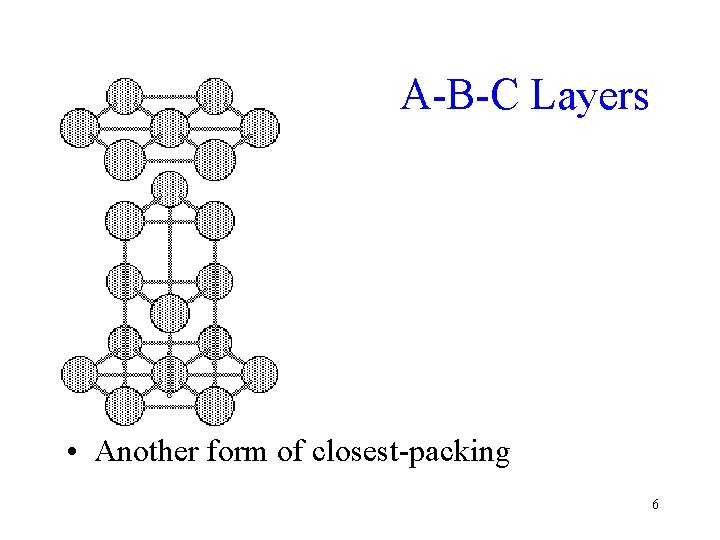
A-B-C Layers • Another form of closest-packing 6

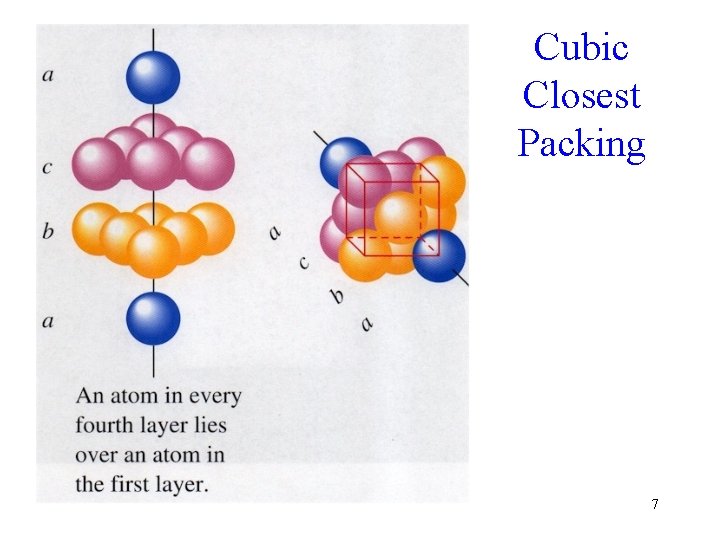
Cubic Closest Packing 7

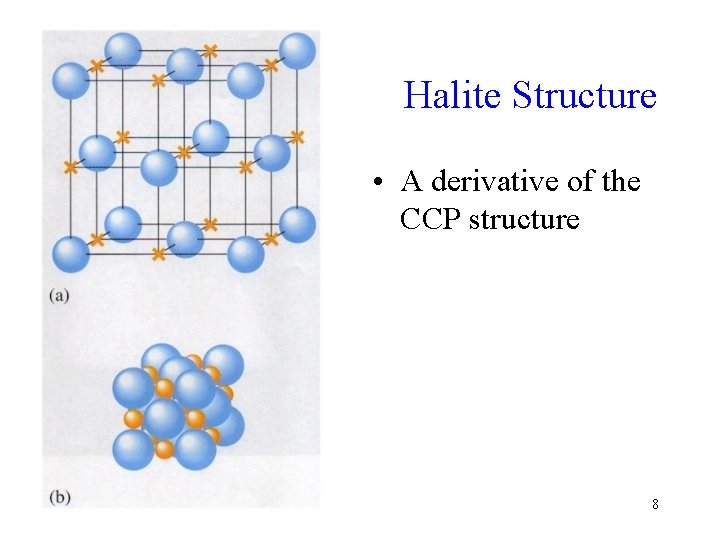
Halite Structure • A derivative of the CCP structure 8

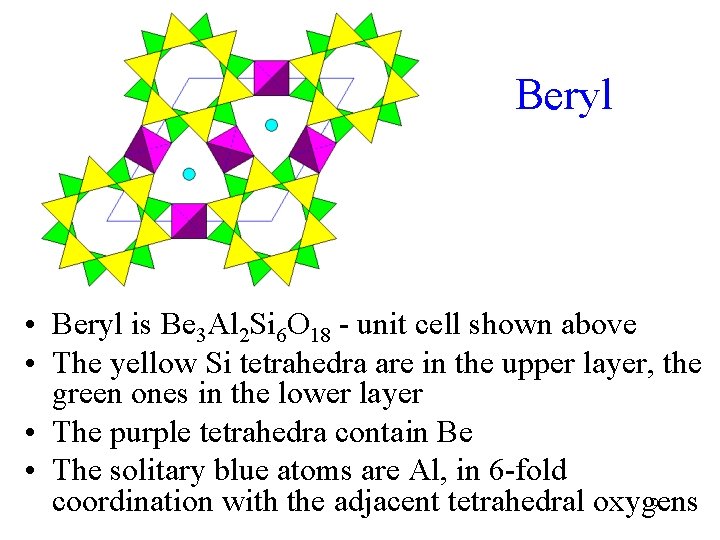
Beryl • Beryl is Be 3 Al 2 Si 6 O 18 - unit cell shown above • The yellow Si tetrahedra are in the upper layer, the green ones in the lower layer • The purple tetrahedra contain Be • The solitary blue atoms are Al, in 6 -fold 9 coordination with the adjacent tetrahedral oxygens

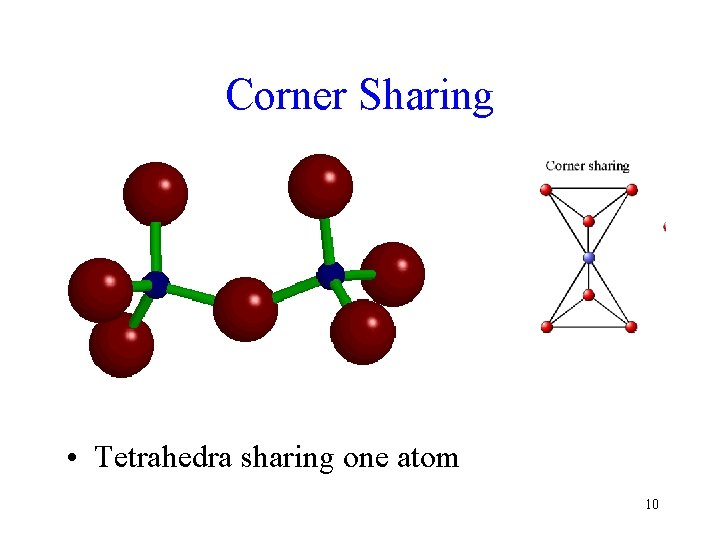
Corner Sharing • Tetrahedra sharing one atom 10

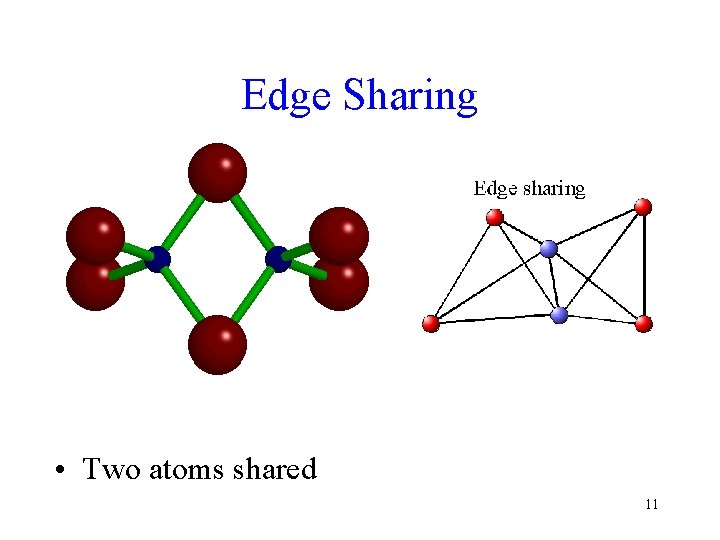
Edge Sharing • Two atoms shared 11

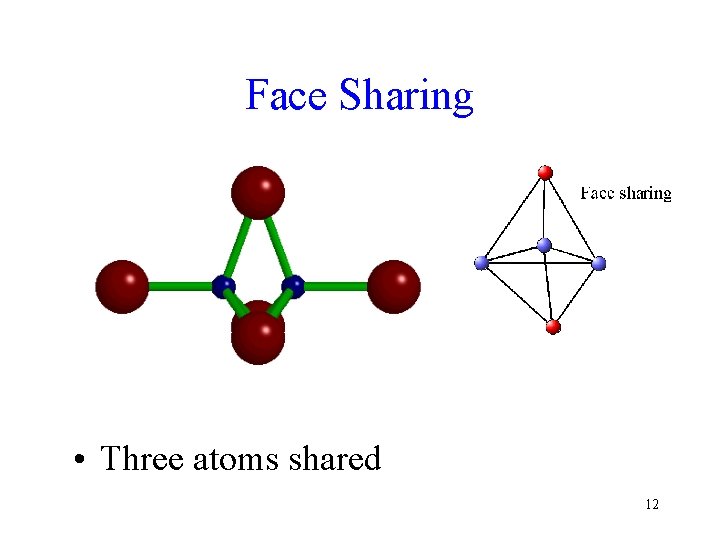
Face Sharing • Three atoms shared 12

Linus Pauling 1901 -1994 • One of four people who have been the recipient of two Nobel Prizes, the prize for chemistry in 1954 and the peace prize in 1960 • During his long life, he was engaged in a broad range of interests within the sciences and, along with many of his colleagues in the scientific community, was actively involved in the antinuclear bomb movement after World War II • Photograph by Michael 13 Collopy

Rule 1 • A coordinated polyhedron of anions is formed about each cation, the cationanion distance being determined by the radius sum, and the coordination number of the cation by the radius ratio 14

Rule 2 • Sometimes called “The electrostatic valiancy principle” • In a stable ionic structure, the valence of each anion, with changed sign, is exactly or nearly equal to the sum of the strengths of the electrostatic bonds to it from the adjacent cations 15

Rule 3 • The existence of edges, and particularly of faces, common to two anion polyhedra in a coordinated structure decreases its stability; this effect is large for cations with high valency and small coordination number, and is especially large when the radius ratio approaches the lower limit of stability of the polyhedron 16

Rule 4 • In a crystal containing different cations, those of high valency and small coordination number tend not to share polyhedron elements with each other 17

Rule 5 • “The principle of parsimony” • The number of essentially different kinds of constituents in a crystal tends to be small 18

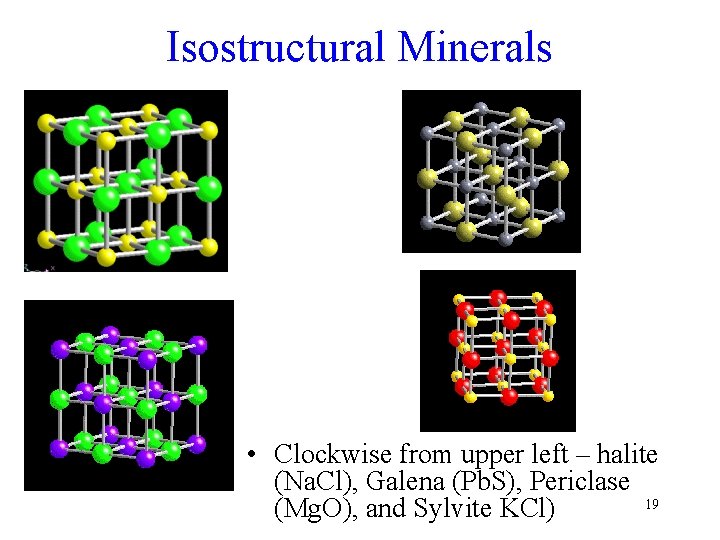
Isostructural Minerals • Clockwise from upper left – halite (Na. Cl), Galena (Pb. S), Periclase 19 (Mg. O), and Sylvite KCl)