Paulings rules Paulings Rules Paulings first rule states




















- Slides: 33


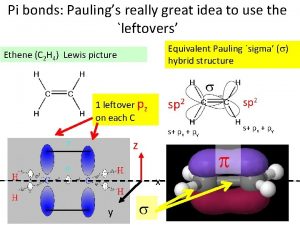
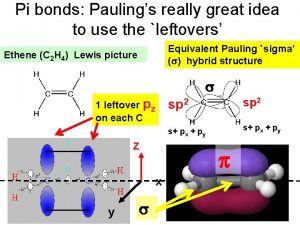
Pauling’s rules

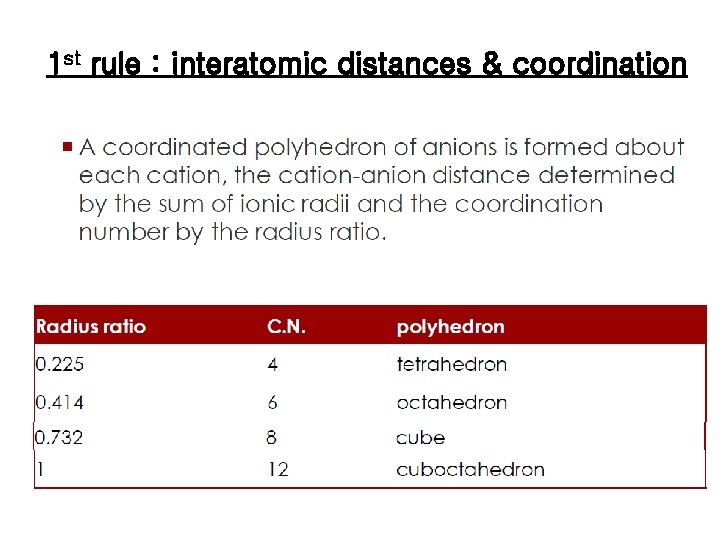
Pauling’s Rules Pauling’s first rule states that coordination polyhedra are formed. Coordination polyhedra are three-dimensional geometric constructions such as tetrahedra and octahedra. Which polyhedron will form is related to the radii of the anions and cations in the compound Pauling’s second rule on the packing of ions states that local electrical neutrality is maintained Pauling’s third rule tells us how to link these polyhedra together. Pauling’s fourth rule is similar to the third, stating that polyhedra formed about cations of low coordination number and high charge tend to be linked by corners. The fifth and final rule states that the number of different constituents in a structure tends to be small; that is, it is difficult to efficiently pack differentsized polyhedra into a single structure.

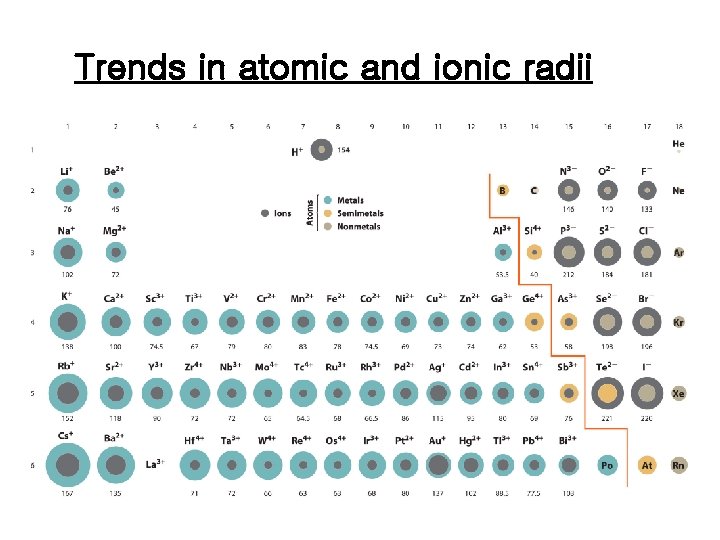
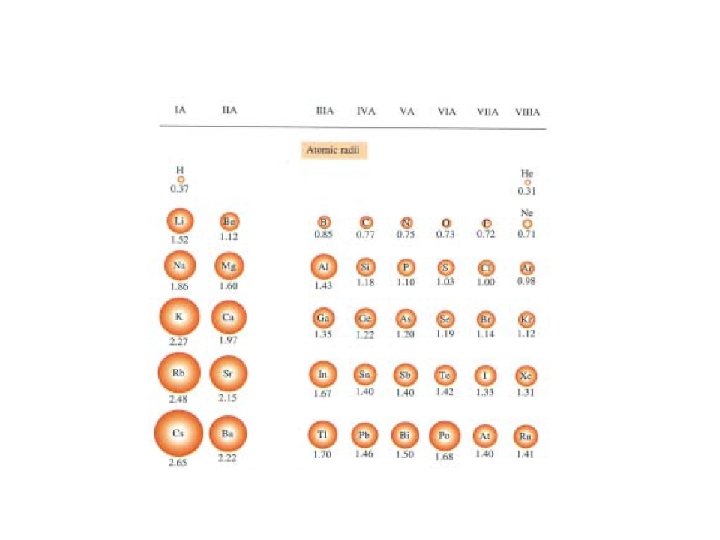
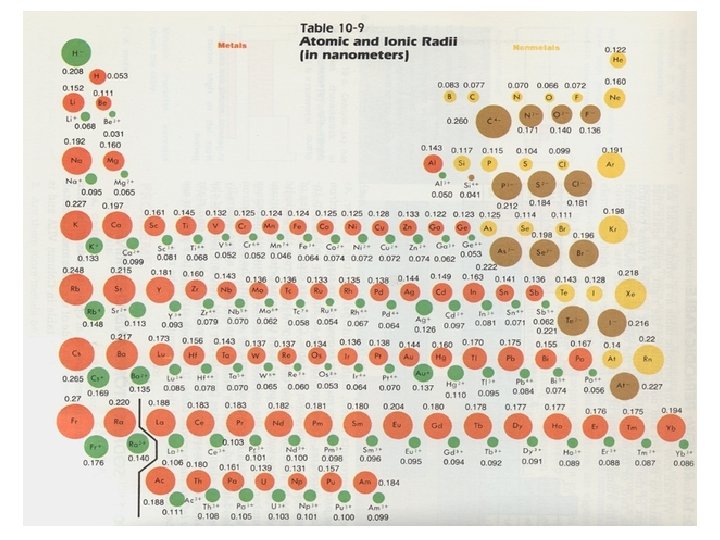
Atomic and ionic radii

Trends in atomic and ionic radii



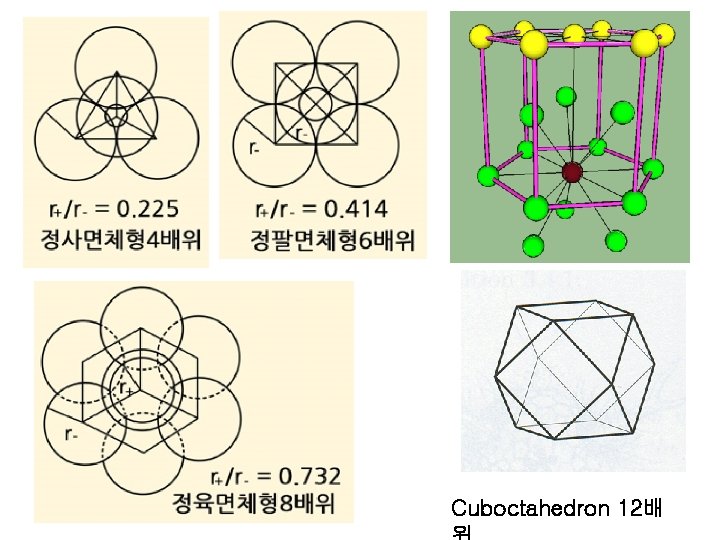
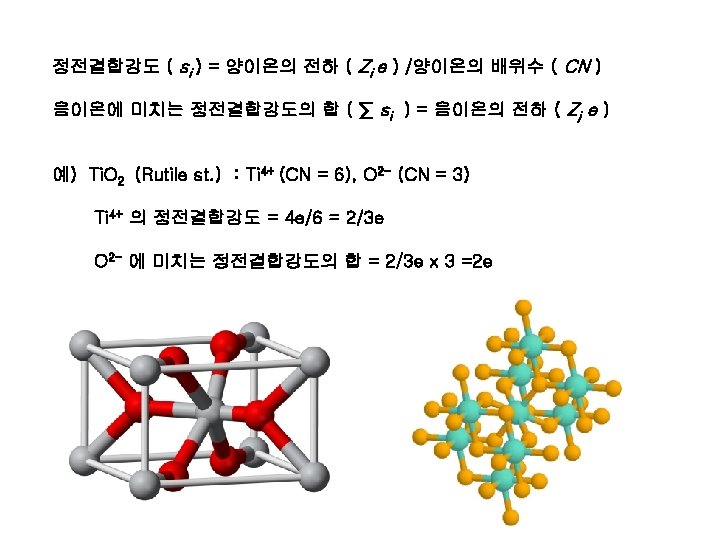
1 st rule : interatomic distances & coordination

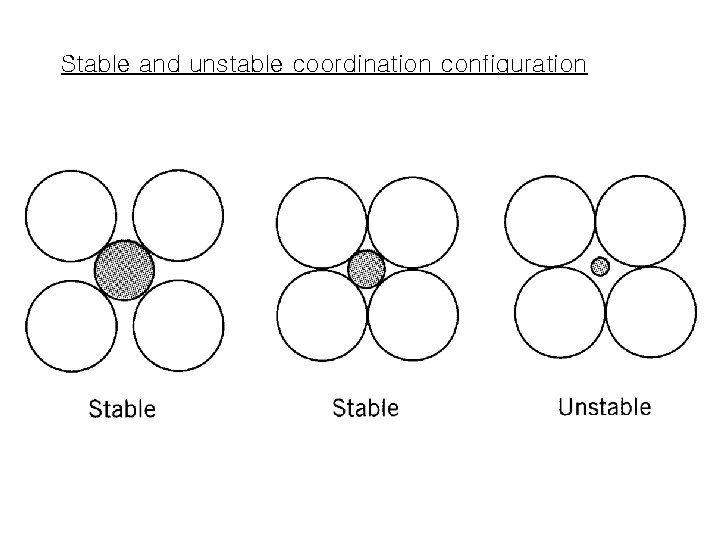
Stable and unstable coordination configuration

Cuboctahedron 12배



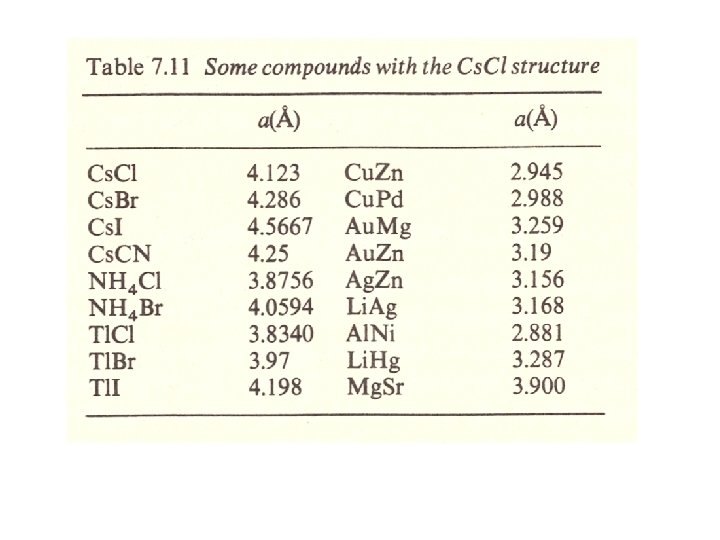
2 nd rule : the electrostatic valency rule


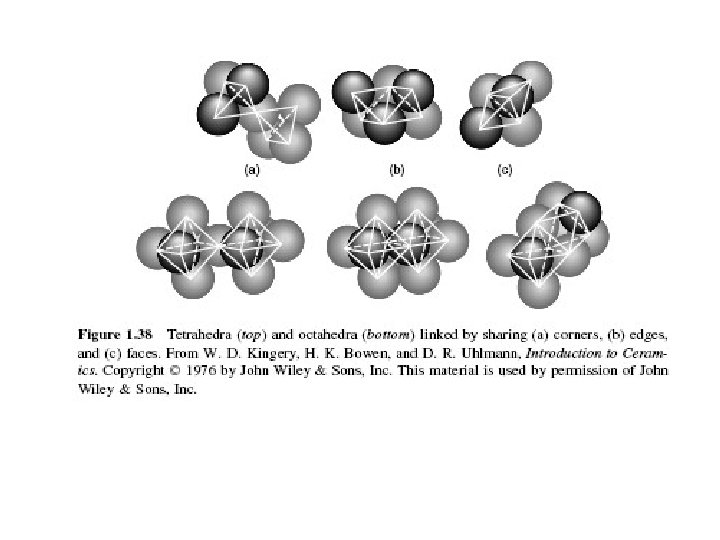
3 rd rule : Sharing polyhedra (1) ØAnion polyhedra that share edges or faces reduce the stability of a crystal because they bring cations closer together. The closer cations experience more electrostatic repulsion.




4 th rule : Sharing polyhedra (2) ØPauling’s fourth rule says that highly charged cations will tend to be as far apart as possible to minimize electrostatic repulsion.


5 th rule : The rule of Parsimony Ø The same cations in a crystal structure have the same coordination number for the same anions.







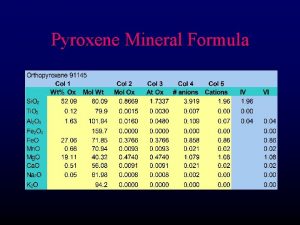
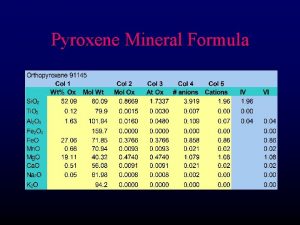
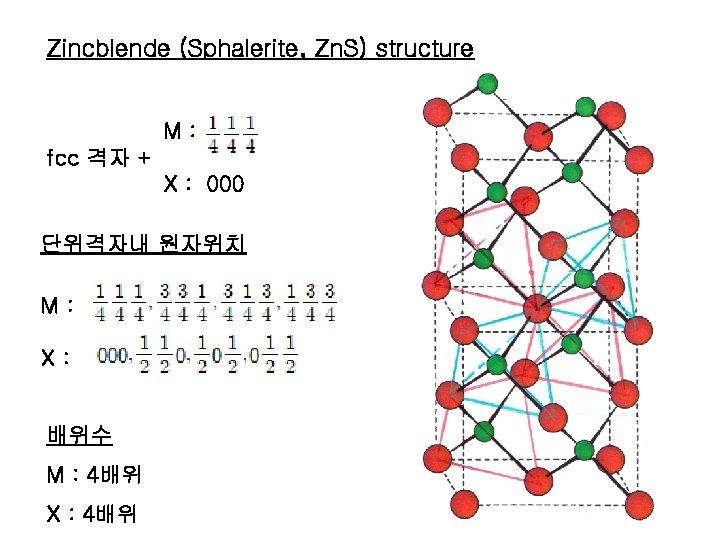
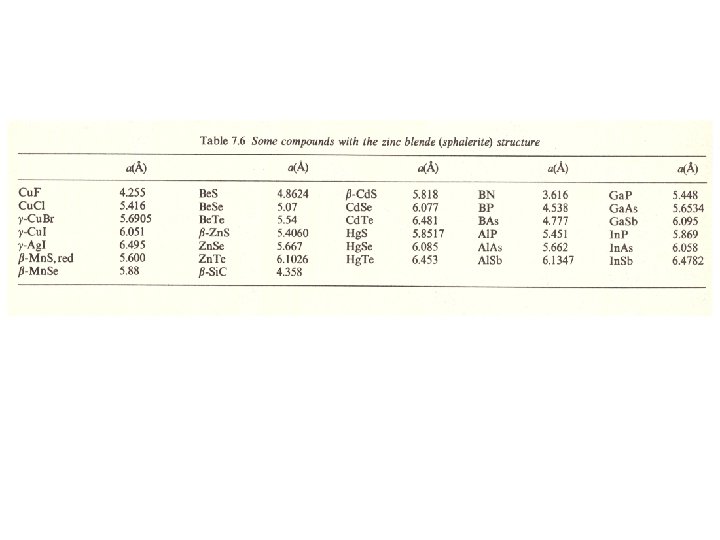
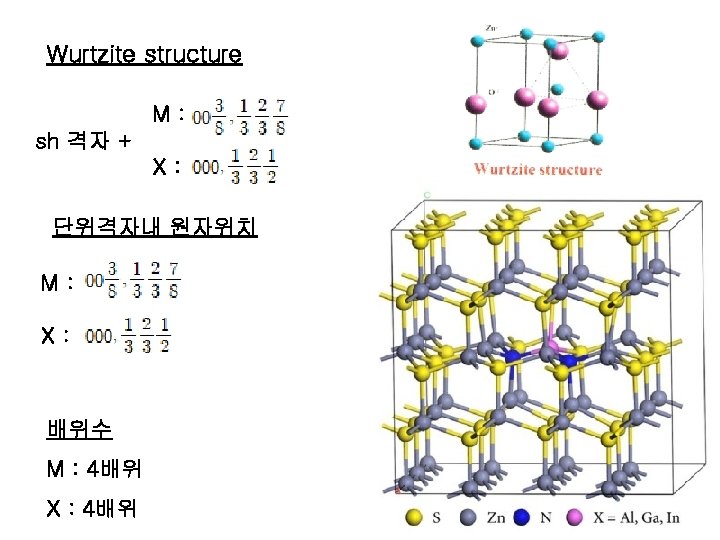
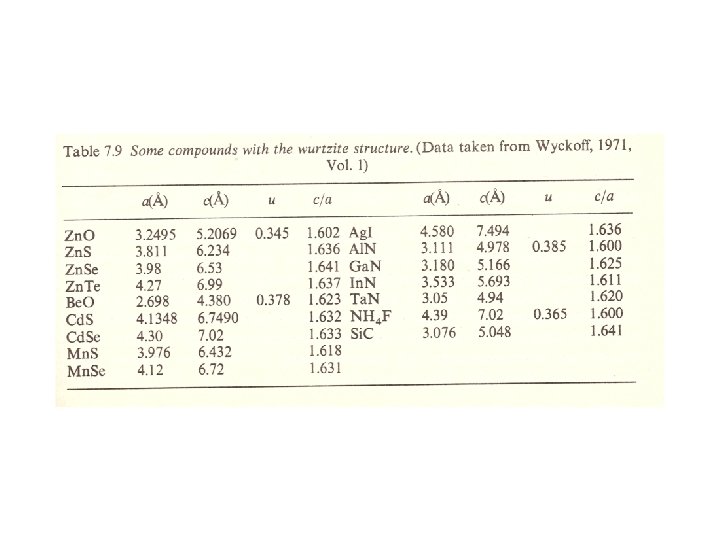
Zincblende (Sphalerite, Zn. S) structure M: fcc 격자 + X : 000 단위격자내 원자위치 M: X: 배위수 M : 4배위 X : 4배위





 Slave states free states
Slave states free states Southern states vs northern states
Southern states vs northern states Big states vs small states guard against tyranny
Big states vs small states guard against tyranny The octet rule states that
The octet rule states that Octet rule
Octet rule What's a chemical bond
What's a chemical bond Bent molecular shape
Bent molecular shape The octet rule states that
The octet rule states that The junction rule
The junction rule What is the sine and cosine rule
What is the sine and cosine rule Basic principle of fingerprint
Basic principle of fingerprint First 13 states
First 13 states Magic triangles trigonometry
Magic triangles trigonometry Sine rule and cosine rule
Sine rule and cosine rule Product rule for counting examples
Product rule for counting examples Dillon rule
Dillon rule Kirchhoff's loop law equation
Kirchhoff's loop law equation Bi oblique astigmatism
Bi oblique astigmatism With the-rule astigmatism example
With the-rule astigmatism example Astigmatism classification
Astigmatism classification Product rule
Product rule General power rule
General power rule Sine and cosine rules
Sine and cosine rules Chain rule power rule
Chain rule power rule Tukey's rule vs empirical rule
Tukey's rule vs empirical rule Social etiquette and manners jrotc
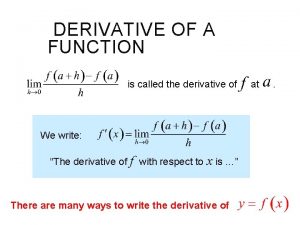
Social etiquette and manners jrotc Derivative of the function
Derivative of the function First order rule learning in machine learning
First order rule learning in machine learning What is factoring
What is factoring Rules in filling up the apartment aufbau principle
Rules in filling up the apartment aufbau principle Rules in filling up the apartment hund's rule
Rules in filling up the apartment hund's rule Maturity continuum examples
Maturity continuum examples Recursive breadth first search
Recursive breadth first search Sdl first vs code first
Sdl first vs code first