Hang posters Then review video Electrons ATOMIC ORBITALS

Hang posters. Then review video
Electrons

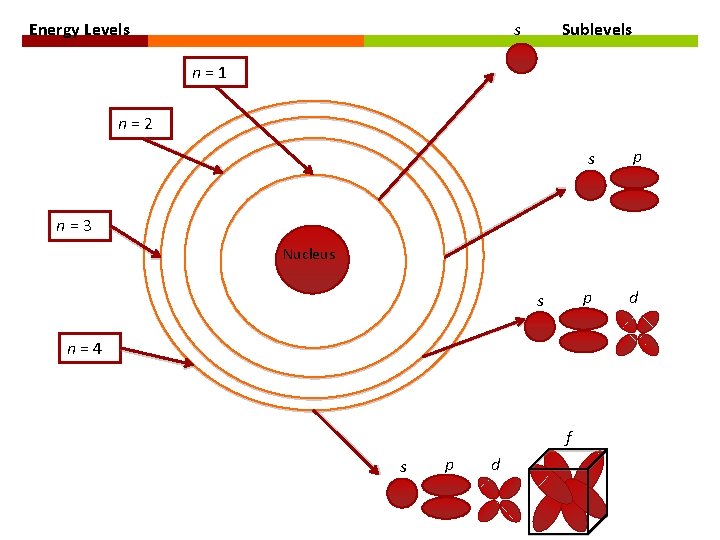
ATOMIC ORBITALS Energy levels: Electrons are located in energy levels. Each energy level is a specific distance from the nucleus. Atomic Orbital: region of space within each energy level in which there is a high probability of finding an electron.
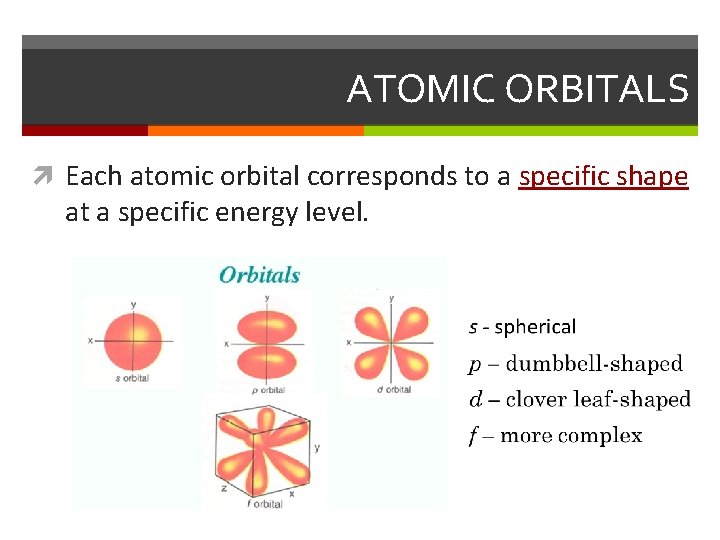
ATOMIC ORBITALS Each atomic orbital corresponds to a specific shape at a specific energy level.

ENERGY LEVELS & SUBLEVELS Principle Energy Level Number of Sublevels Type of Sublevel n=1 1 1 s (1 orbital) n=2 2 2 s (1 orbital), 2 p (3 orbitals) n=3 3 3 s (1 orbital), 3 p (3 orbitals), 3 d (5 orbitals) n=4 4 4 s (1 orbital), 4 p (3 orbitals), 4 d (5 orbitals), 4 f (7 orbitals)
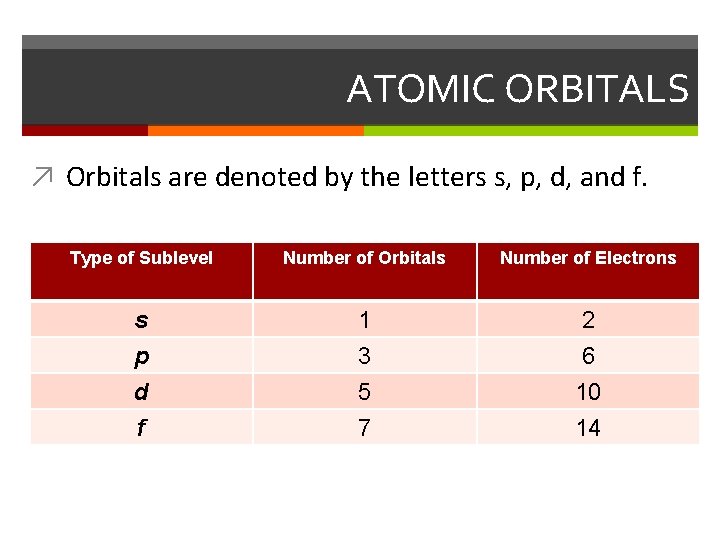
ATOMIC ORBITALS ↗ Orbitals are denoted by the letters s, p, d, and f. Type of Sublevel Number of Orbitals Number of Electrons s p 1 3 2 6 d f 5 7 10 14
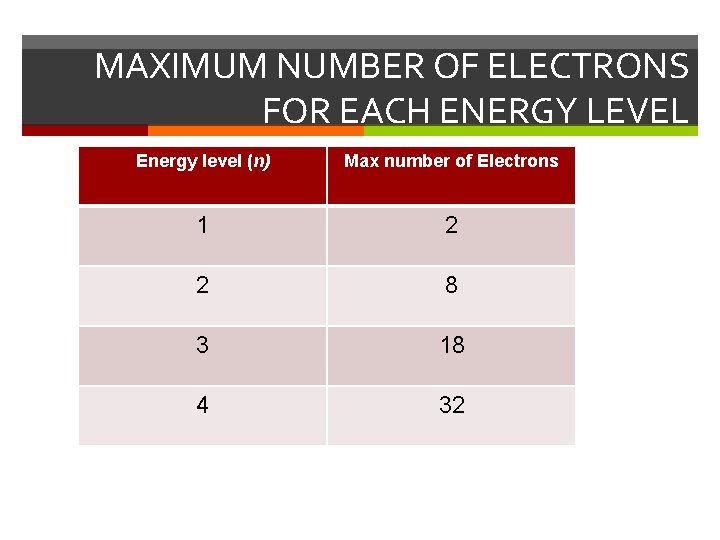
MAXIMUM NUMBER OF ELECTRONS FOR EACH ENERGY LEVEL Energy level (n) Max number of Electrons 1 2 2 8 3 18 4 32
Energy Levels s Sublevels n=1 n=2 s p p d n=3 Nucleus s n=4 f s p d
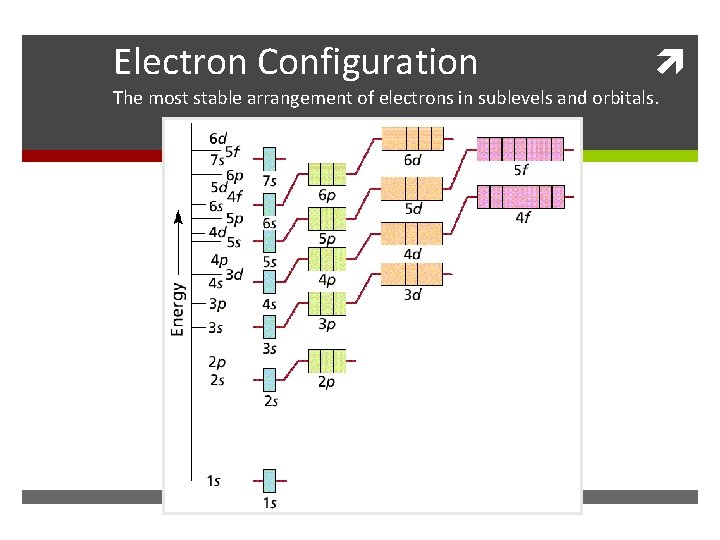
Electron Configuration The most stable arrangement of electrons in sublevels and orbitals.
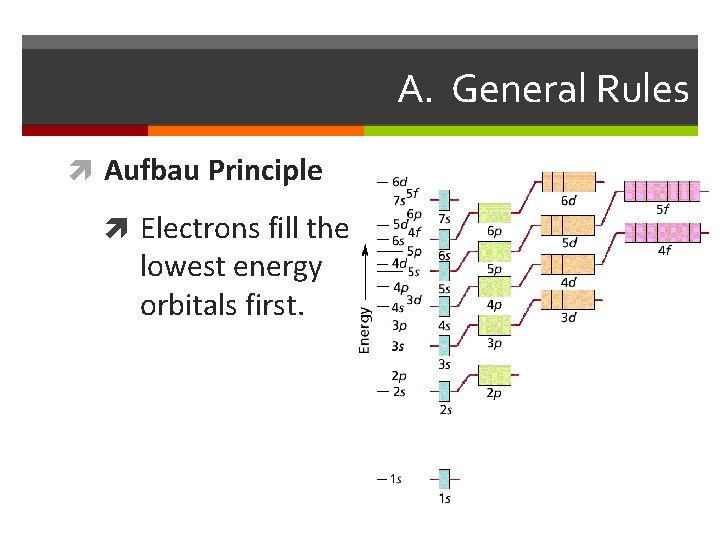
A. General Rules Aufbau Principle Electrons fill the lowest energy orbitals first.
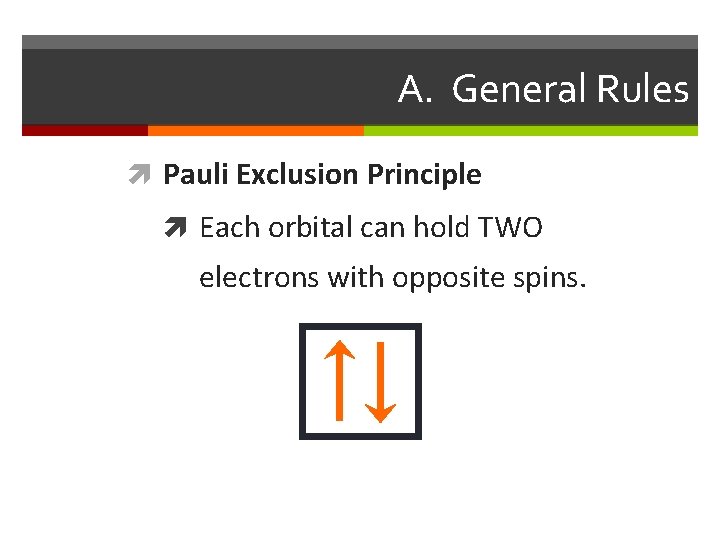
A. General Rules Pauli Exclusion Principle Each orbital can hold TWO electrons with opposite spins.
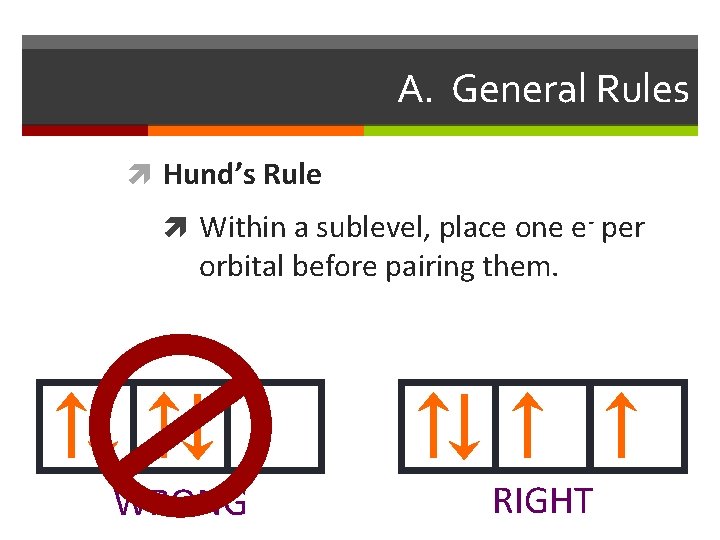
A. General Rules Hund’s Rule Within a sublevel, place one e- per orbital before pairing them. WRONG RIGHT

Apartment Rules Electron Rules From the Bottom Up: Rooms must be filled from the Aufbau Principle: the electrons fill the available orbitals ground floor up. Fill the one room on the first floor before from lowest energy to highest energy. In the ground state starting to put new tenants on the second floor. Then fill all the electrons are in the lowest possible energy level. the s room before the p rooms. At higher floors the order might change a bit. Singles First: the owner of the building wants to have the tenants spread out as much as possible. For that reason singles are placed in rooms before couples. If couples must be placed into a room then all of the other rooms on that floor must already have a single in them. Hund’s Rule: The electrons must be placed into the orbitals in such a way that no pairs are put together unless absolutely necessary. That is, single electrons must be placed into boxes first and then paired up if necessary. Opposite Gender Only: When two people are placed in a Pauli Exclusion Principle: Electrons come in two varieties room they must be of opposite genders. No men may based on the direction they are ‘spinning’. There is an Up room together and no women may room together. This is spin and a Down spin. Up and Down spins are always an arbitrary rule on the part of the owners: in a just world paired together and Up-Up or Down-Down combinations we wouldn’t have to follow it. But quantum mechanics has are not allowed. No two electrons can ever be in the same nothing to do with justice. place at the same time.
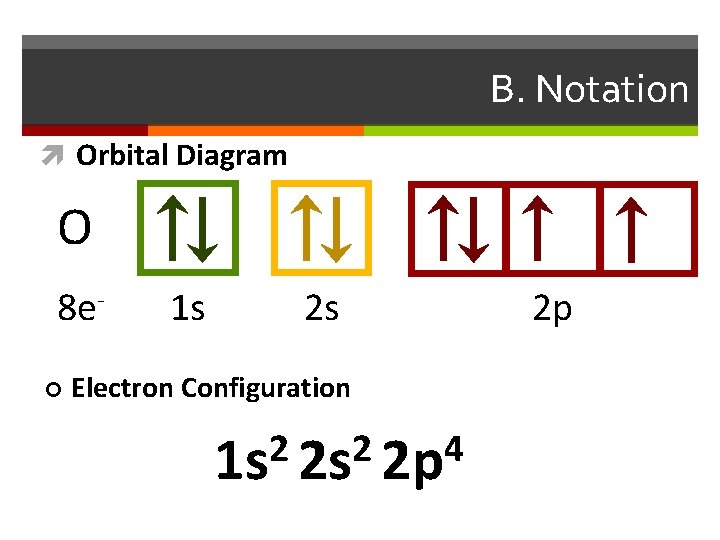
B. Notation Orbital Diagram O 8 e¢ 1 s 2 s Electron Configuration 2 2 4 1 s 2 s 2 p 2 p
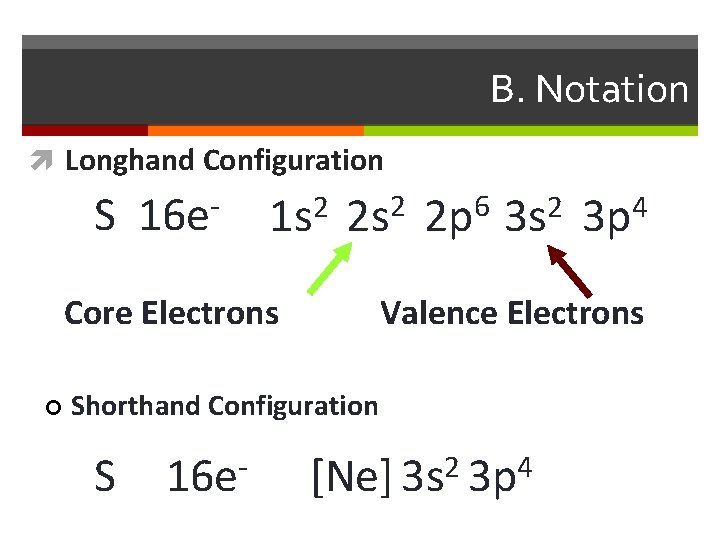
B. Notation Longhand Configuration S 16 e 1 s 2 2 p 6 3 s 2 3 p 4 Core Electrons ¢ Valence Electrons Shorthand Configuration S 16 e- [Ne] 3 s 2 3 p 4
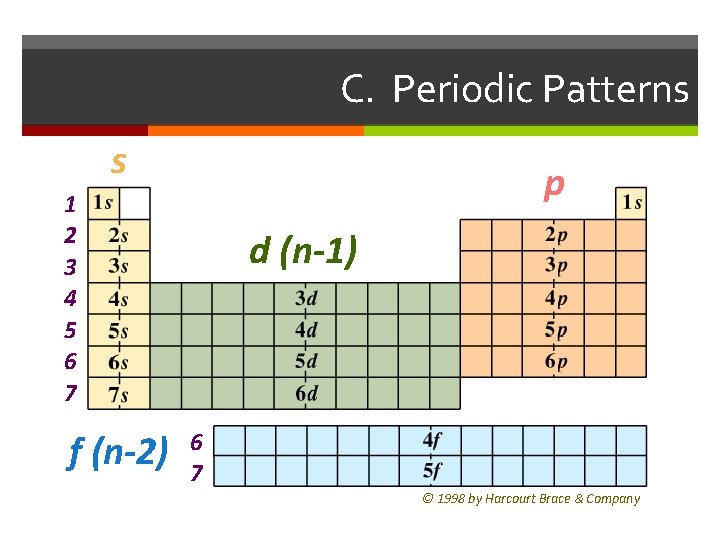
C. Periodic Patterns s p 1 2 3 4 5 6 7 f (n-2) d (n-1) 6 7 © 1998 by Harcourt Brace & Company
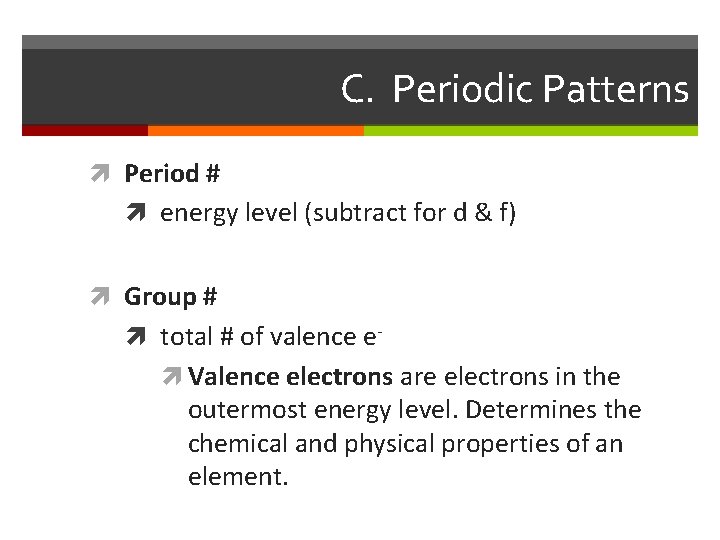
C. Periodic Patterns Period # energy level (subtract for d & f) Group # total # of valence e Valence electrons are electrons in the outermost energy level. Determines the chemical and physical properties of an element.

Wednesday. . 9 -28 -16 Get out your notes from yesterday…. HMWK: Work on HMWK packet… due Monday with test on Unit 3
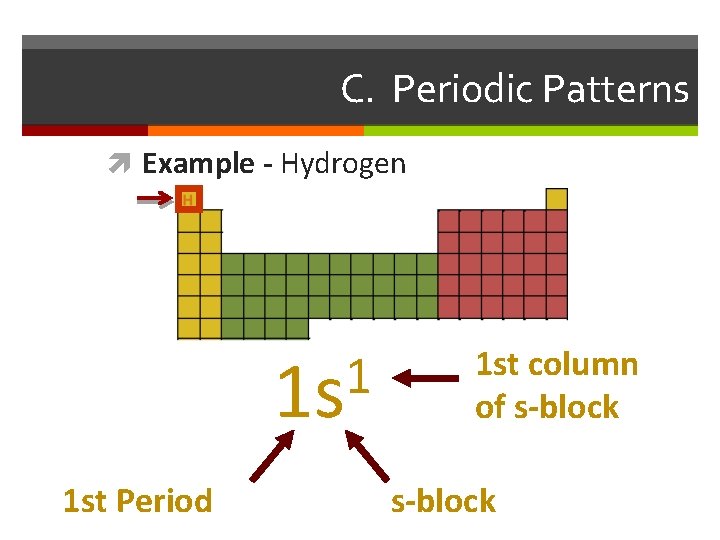
C. Periodic Patterns Example - Hydrogen 1 1 s 1 st Period 1 st column of s-block
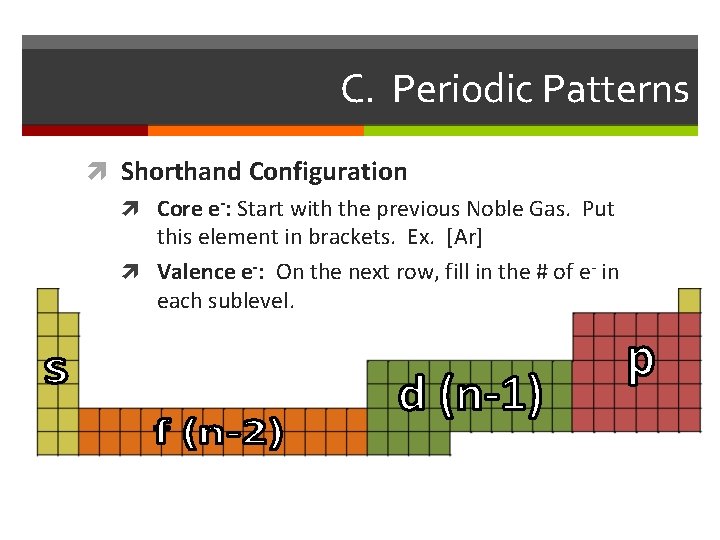
C. Periodic Patterns Shorthand Configuration Core e-: Start with the previous Noble Gas. Put this element in brackets. Ex. [Ar] Valence e-: On the next row, fill in the # of e- in each sublevel.
![C. Periodic Patterns Example - Germanium [Ar] 2 4 s 10 3 d 2 C. Periodic Patterns Example - Germanium [Ar] 2 4 s 10 3 d 2](http://slidetodoc.com/presentation_image_h/0e591f7b0b8823f3875ab4c1dff0a012/image-21.jpg)
C. Periodic Patterns Example - Germanium [Ar] 2 4 s 10 3 d 2 4 p
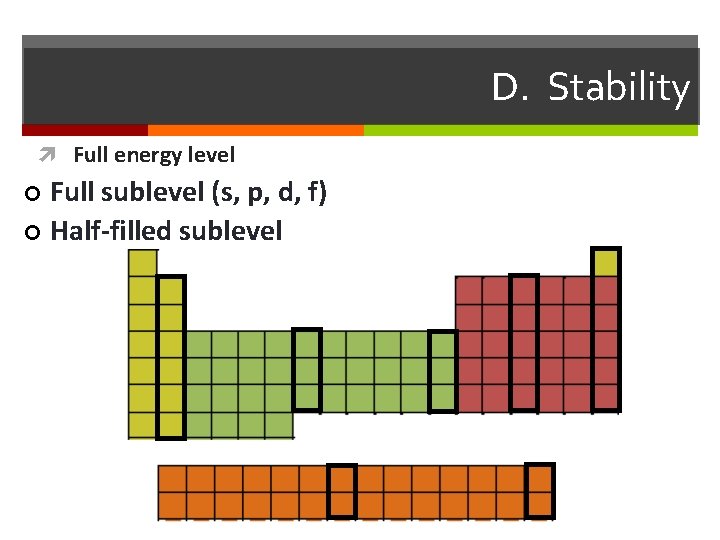
D. Stability Full energy level Full sublevel (s, p, d, f) ¢ Half-filled sublevel ¢
![D. Stability Electron Configuration Exceptions l l Copper EXPECT: [Ar] 4 s 2 3 D. Stability Electron Configuration Exceptions l l Copper EXPECT: [Ar] 4 s 2 3](http://slidetodoc.com/presentation_image_h/0e591f7b0b8823f3875ab4c1dff0a012/image-23.jpg)
D. Stability Electron Configuration Exceptions l l Copper EXPECT: [Ar] 4 s 2 3 d 9 ACTUALLY: [Ar] 4 s 1 3 d 10 Copper gains stability with a full d-sublevel.
![D. Stability Electron Configuration Exceptions l Chromium EXPECT: ACTUALLY: l [Ar] 4 s 2 D. Stability Electron Configuration Exceptions l Chromium EXPECT: ACTUALLY: l [Ar] 4 s 2](http://slidetodoc.com/presentation_image_h/0e591f7b0b8823f3875ab4c1dff0a012/image-24.jpg)
D. Stability Electron Configuration Exceptions l Chromium EXPECT: ACTUALLY: l [Ar] 4 s 2 3 d 4 [Ar] 4 s 1 3 d 5 Chromium gains stability with a half-filled dsublevel.
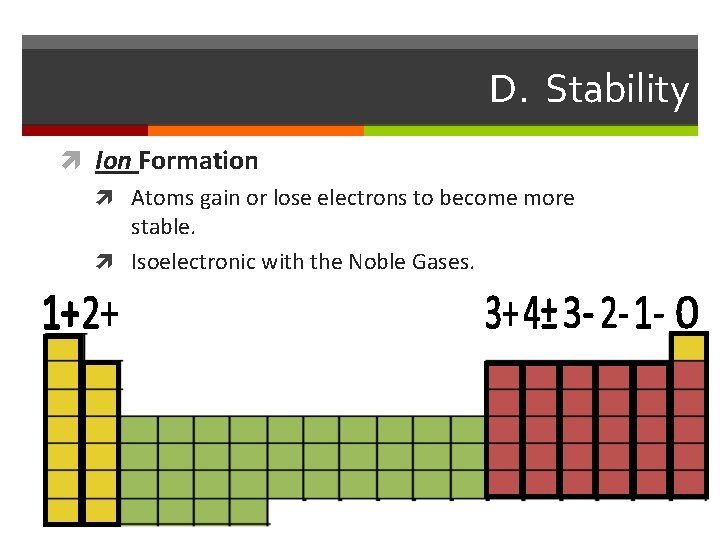
D. Stability Ion Formation Atoms gain or lose electrons to become more stable. Isoelectronic with the Noble Gases.
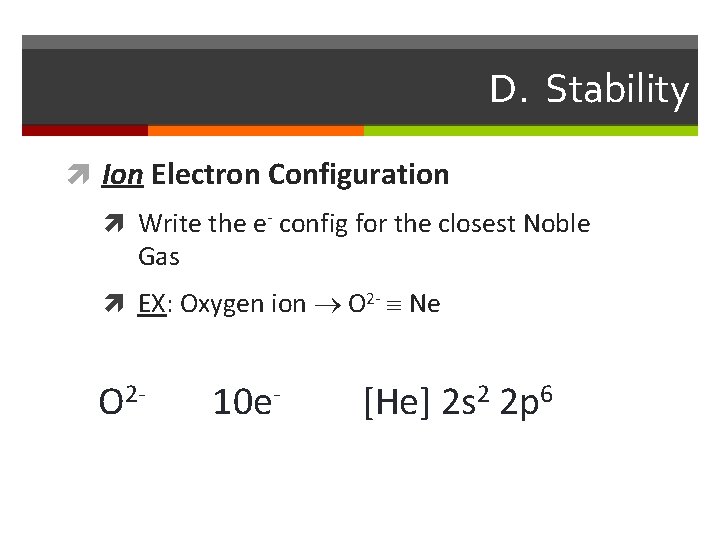
D. Stability Ion Electron Configuration Write the e- config for the closest Noble Gas EX: Oxygen ion O 2 - Ne O 2 - 10 e- [He] 2 s 2 2 p 6

Thursday… 9 -29 -16 If you have a smiley face and check plus on your interim, you may leave at the bell If you are retesting Unit 2…. You need to hand me a completed remediation sheet you printed off the weebly

Friday… 9 -30 -16 Get out your homework packet… work quietly on it Test is Monday…. . HMWK: Finish packet. Work on HP part 3– due October 14 th

Thursday…. 9 -29 -16 Put your classwork/ book work in the “In” box…. . And get with a partner you want to WORK with HMWK: Test Monday on Unit 3 Periodic Table… Homework Packet due also HP part 3– Interview due Oct. 14 th

Monday… 10 -3 -16 Get out your completed Homework packet and review quietly for your Unit 3 test. HMWK: Work on HP Part 3… due Oct. 14 th
- Slides: 30