P K CHOURASIA PRESENTS SURFACE CHEMISTRY Surface Chemistry





- Slides: 26

P. K CHOURASIA PRESENTS • SURFACE CHEMISTRY

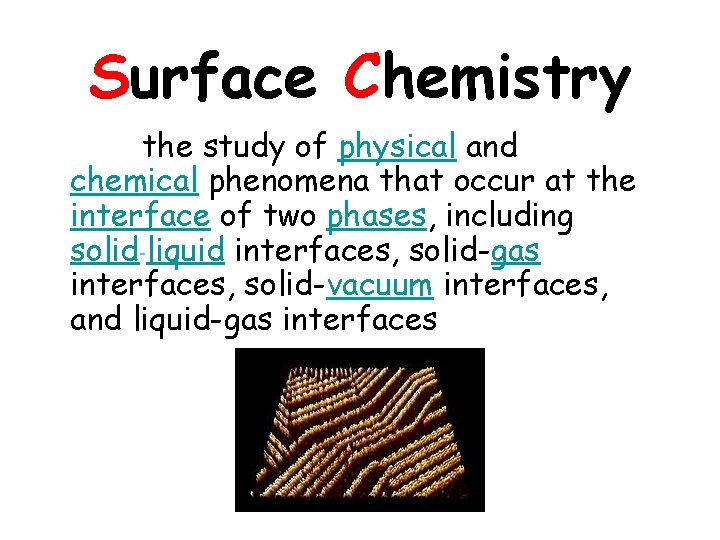
Surface Chemistry the study of physical and chemical phenomena that occur at the interface of two phases, including solid-liquid interfaces, solid-gas interfaces, solid-vacuum interfaces, and liquid-gas interfaces

Phenomena in Surface Chemistry 1. 2. 3. 4. Wetting, Spreading and Penetration Foam Breaking in Aqueous Systems Solubilization Rheological Effects in Surfactant Phases

Assignment-6 Group Presentation G 9 : Foam breaking and its application G 10 : Solubilization and its application G 11 : Rheology and its application (5 min)

Surface Chemistry in Important Technologies § § § Surface Chemistry in Pharmacy Surface Chemistry in Food and Feed Surface Chemistry in Detergency Surface Chemistry in Agriculture Surface and Colloid Chemistry in Photographic Technology Surface Chemistry in Paints Surface Chemistry of Paper Surface Chemistry in the Polymerization of Emulsion Colloidal Processing of Ceramics Surface Chemistry in Dispersion, Flocculation and Flotation Surface Chemistry in the Petroleum Industry

Assignment-7 Individual Report 1. Search from Journals or websites to find some examples of Surface Chemistry in Important Technologies 2. Write up a 1 page concise report in A 4 paper. 3. Report is due on September 15, 2009. (10 points)

Analysis and Characterization in Surface Chemistry Measuring Equilibrium Surface Tensions Measuring Dynamic Surface Tensions Measuring Contact Angle Determining Critical Micelle Concentration Measuring Micelle Size and Shape Identification of Lyotropic Liquid Crystalline Mesophases Characterization of Microemulsion Structure Measuring Particle Size by Light Scattering Measurement of Electrokinetic Phenomena in Surface Chemistry § Measuring Interactions between Surfaces § Measuring the Forces and Stability of Thin-Liquid Films § Measuring Adsorption § § § § §

1. Gas-Liquid and Liquid-liquid Interfaces

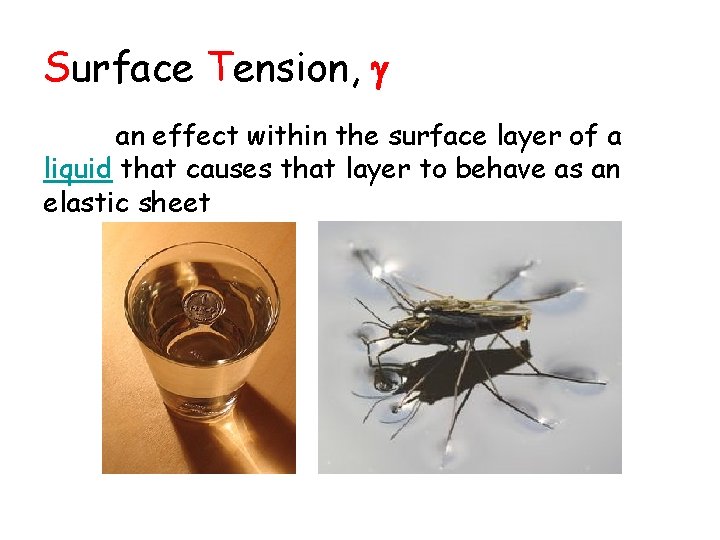
Surface Tension, an effect within the surface layer of a liquid that causes that layer to behave as an elastic sheet

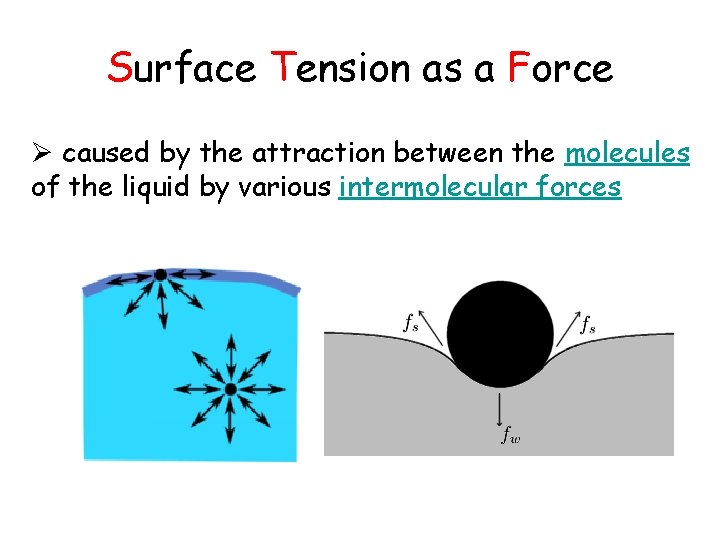
Surface Tension as a Force Ø caused by the attraction between the molecules of the liquid by various intermolecular forces

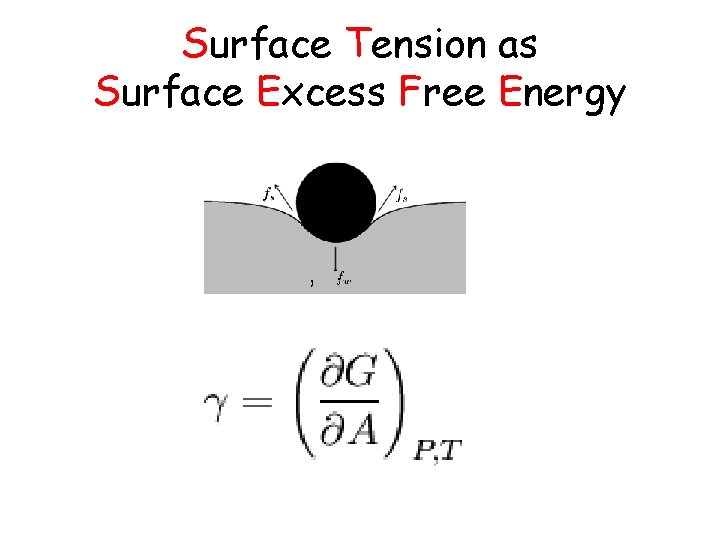
Surface Tension as Surface Excess Free Energy , .

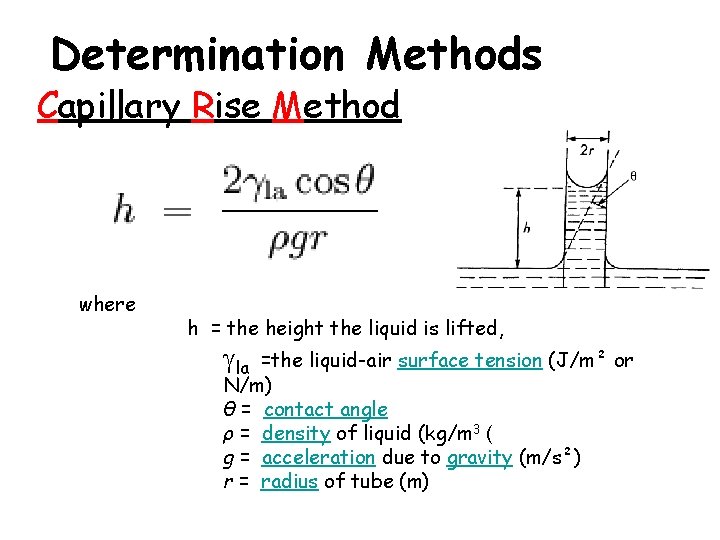
Determination Methods Capillary Rise Method where h = the height the liquid is lifted, la =the liquid-air surface tension (J/m² or N/m) θ = contact angle ρ = density of liquid (kg/m 3 ( g = acceleration due to gravity (m/s²) r = radius of tube (m)

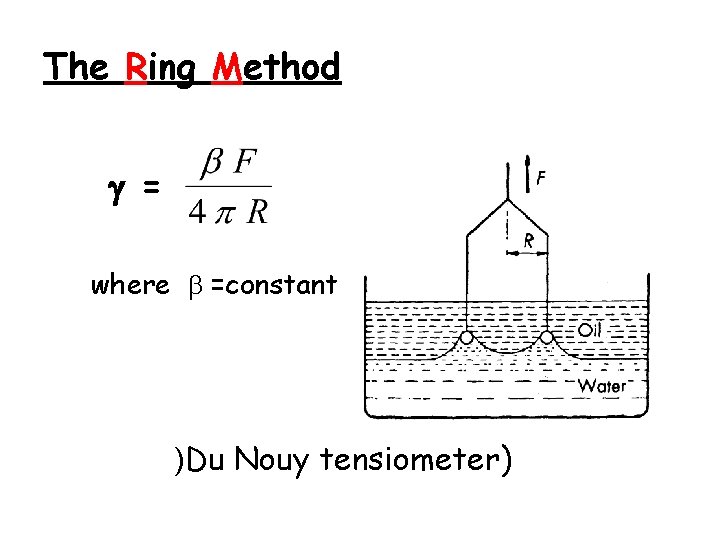
The Ring Method = where =constant )Du Nouy tensiometer)

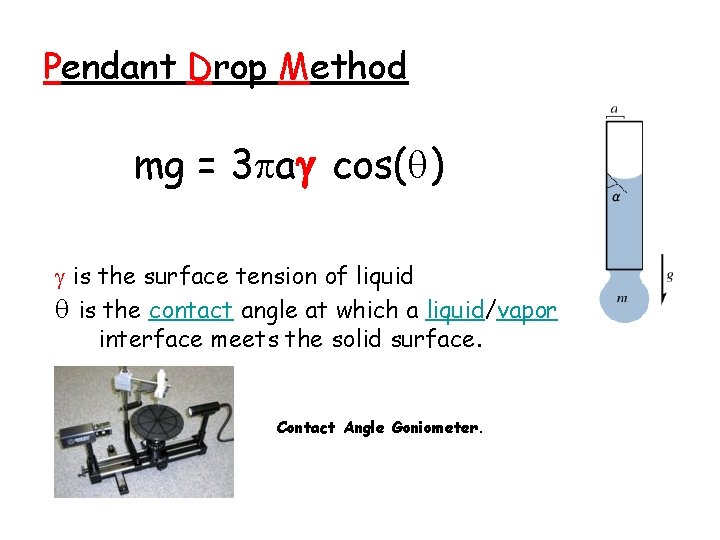
Pendant Drop Method mg = 3 a cos( ) is the surface tension of liquid is the contact angle at which a liquid/vapor interface meets the solid surface. Contact Angle Goniometer.

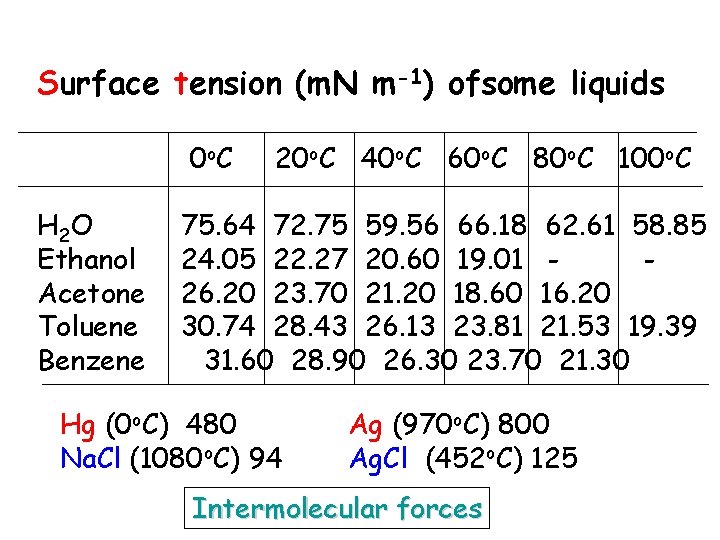
Surface tension (m. N m-1) ofsome liquids 0 o. C H 2 O Ethanol Acetone Toluene Benzene 20 o. C 40 o. C 60 o. C 80 o. C 100 o. C 75. 64 72. 75 59. 56 66. 18 62. 61 58. 85 24. 05 22. 27 20. 60 19. 01 26. 20 23. 70 21. 20 18. 60 16. 20 30. 74 28. 43 26. 13 23. 81 21. 53 19. 39 31. 60 28. 90 26. 30 23. 70 21. 30 Hg (0 o. C) 480 Na. Cl (1080 o. C) 94 Ag (970 o. C) 800 Ag. Cl (452 o. C) 125 Intermolecular forces

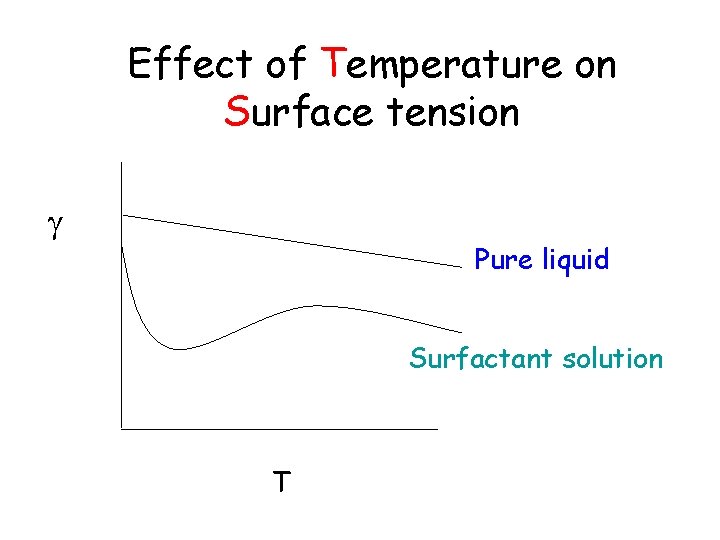
Surface Tension § Directly depends on intermolecular forces in the solution § Inversely depends on temperature § of metallic liquid > ionic liquid > covalent liquid

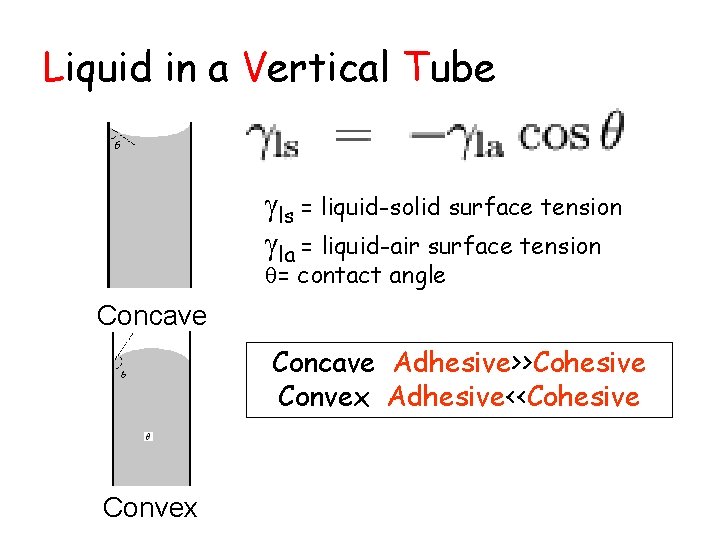
Liquid in a Vertical Tube ls = liquid-solid surface tension la = liquid-air surface tension = contact angle Concave Adhesive>>Cohesive Convex Adhesive<<Cohesive Convex

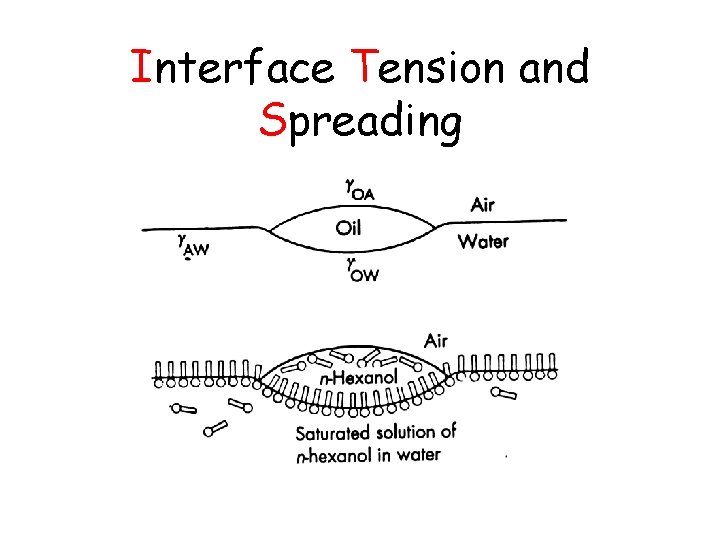
Interface Tension and Spreading

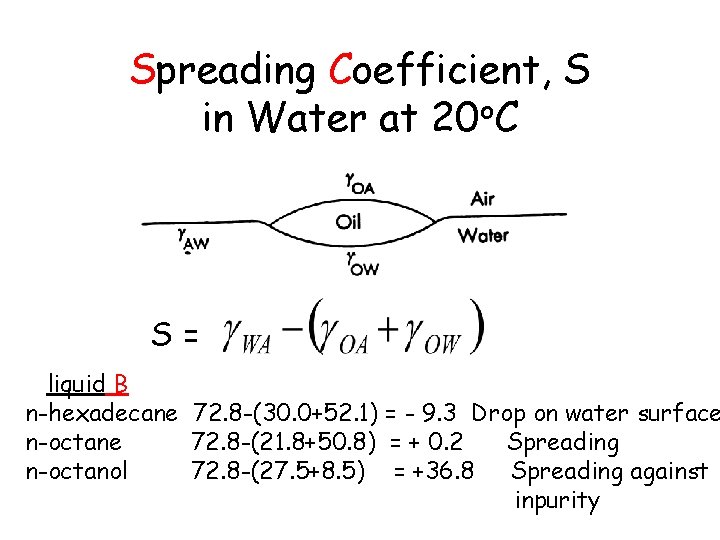
Spreading Coefficient, S in Water at 20 o. C S = S= liquid B n-hexadecane 72. 8 -(30. 0+52. 1) = - 9. 3 Drop on water surface n-octane 72. 8 -(21. 8+50. 8) = + 0. 2 Spreading n-octanol 72. 8 -(27. 5+8. 5) = +36. 8 Spreading against inpurity

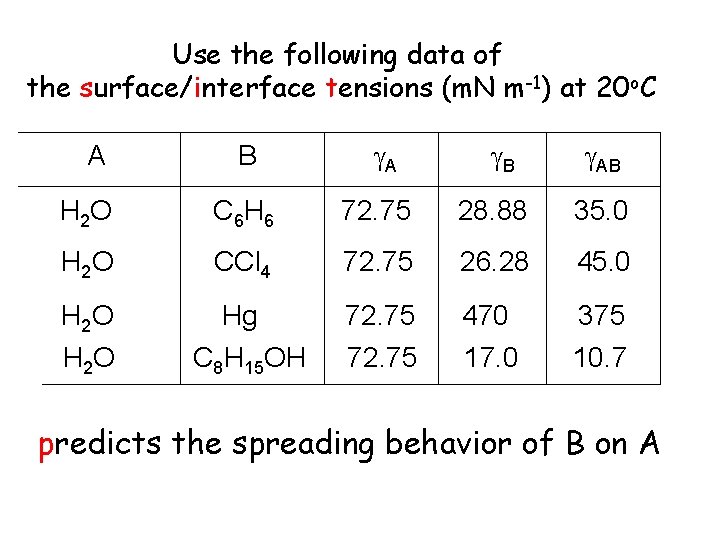
Use the following data of the surface/interface tensions (m. N m-1) at 20 o. C A B AB H 2 O C 6 H 6 72. 75 28. 88 35. 0 H 2 O CCl 4 72. 75 26. 28 45. 0 H 2 O Hg C 8 H 15 OH 72. 75 470 17. 0 375 10. 7 predicts the spreading behavior of B on A

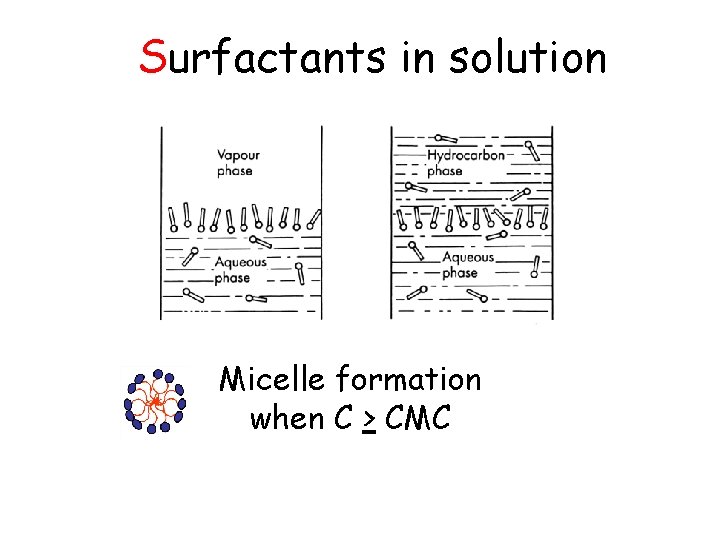
Surface Tension of Solution Substances in solution : q. Surface active agent “Surfactant” ( < o) hydrophilic part hydrophobic part q. Surface inactive agent ( > o) such as ionic compounds, acids, bases etc.

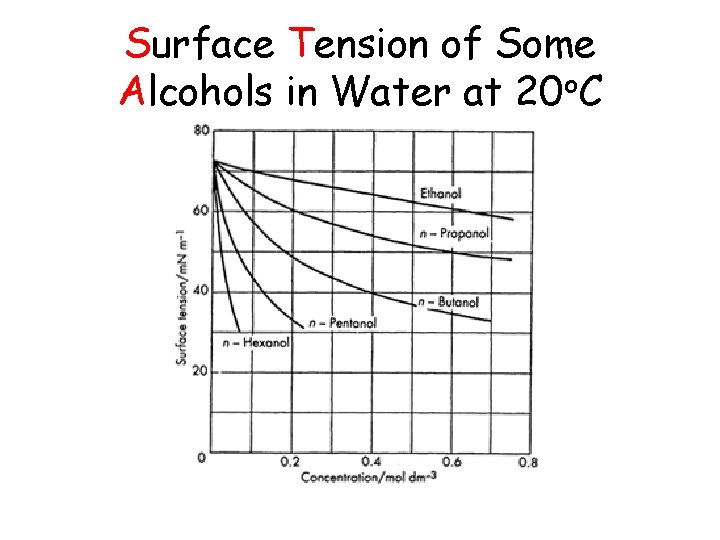
Surface Tension of Some Alcohols in Water at 20 o. C

Surfactants in solution Micelle formation when C > CMC

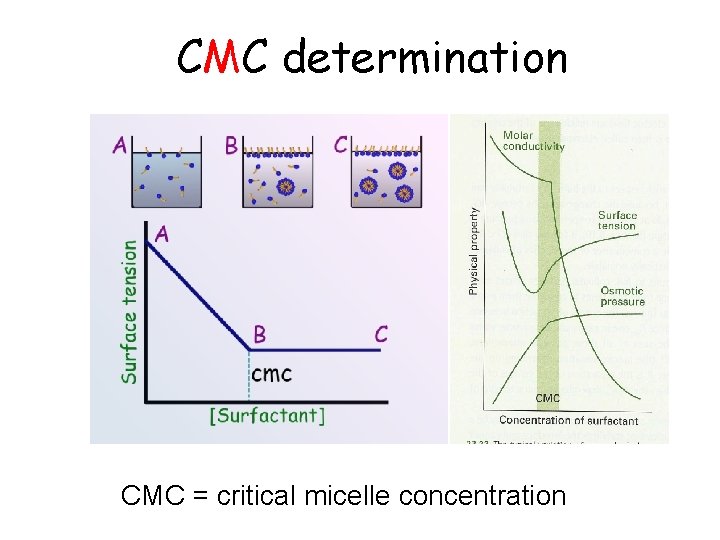
CMC determination CMC = critical micelle concentration

Effect of Temperature on Surface tension Pure liquid Surfactant solution T

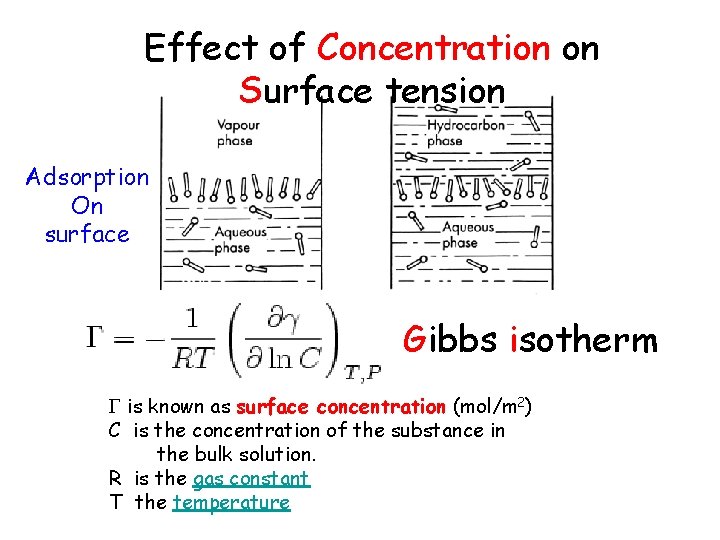
Effect of Concentration on Surface tension Adsorption On surface Gibbs isotherm is known as surface concentration (mol/m 2) C is the concentration of the substance in the bulk solution. R is the gas constant T the temperature
 Gurmit paid £21 for five presents
Gurmit paid £21 for five presents 16 3 darwin presents his case answer key
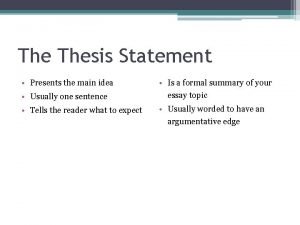
16 3 darwin presents his case answer key A thesis statement presents
A thesis statement presents This presents
This presents Gurmit paid £21 for five presents
Gurmit paid £21 for five presents Presents simple
Presents simple Presents from my aunts in pakistan
Presents from my aunts in pakistan Nollaig shona meaning
Nollaig shona meaning Section 15-3 darwin presents his case answer key
Section 15-3 darwin presents his case answer key Which sentence presents a faulty either/or argument?
Which sentence presents a faulty either/or argument? What is tragedy
What is tragedy Prose writing that presents and explains ideas
Prose writing that presents and explains ideas Is the way an author presents a character.
Is the way an author presents a character. Pear paragraphs
Pear paragraphs A tes pieds ô divin roi nous apportons nos présents
A tes pieds ô divin roi nous apportons nos présents Chapter 17 neurologic emergencies
Chapter 17 neurologic emergencies Zids and zods answers
Zids and zods answers Elements of drama questions
Elements of drama questions Prose writing that presents and explains ideas
Prose writing that presents and explains ideas Which type of communication is shown in this poster?
Which type of communication is shown in this poster? Sally elatta presents on business agility
Sally elatta presents on business agility A 26 year old female presents
A 26 year old female presents Is thesis statement and main idea the same
Is thesis statement and main idea the same Sue palmer skeleton books
Sue palmer skeleton books A text that presents both side of the topic
A text that presents both side of the topic Key words for cause and effect text structure
Key words for cause and effect text structure Inorganic vs organic chemistry
Inorganic vs organic chemistry