p H calculations p H calculations CONTENTS What



![What is p. H? p. H = - log 10 [H+(aq)] where [H+] is What is p. H? p. H = - log 10 [H+(aq)] where [H+] is](https://slidetodoc.com/presentation_image_h2/3a49265d449918f9dd8ee3a0a291561e/image-4.jpg)






- Slides: 17

p. H calculations

p. H calculations CONTENTS • What is p. H? - a reminder • Calculating the p. H of strong acids and bases • Calculating the p. H of weak acids • Calculating the p. H of mixtures - strong acid and strong alkali

p. H calculations Before you start it would be helpful to… • know the differences between strong and weak acid and bases • be able to calculate p. H from hydrogen ion concentration • be able to calculate hydrogen ion concentration from p. H • know the formula for the ionic product of water and its value at 25°C
![What is p H p H log 10 Haq where H is What is p. H? p. H = - log 10 [H+(aq)] where [H+] is](https://slidetodoc.com/presentation_image_h2/3a49265d449918f9dd8ee3a0a291561e/image-4.jpg)
What is p. H? p. H = - log 10 [H+(aq)] where [H+] is the concentration of hydrogen ions in mol dm-3 to convert p. H into hydrogen ion concentration IONIC PRODUCT OF WATER [H+(aq)] = antilog (-p. H) Kw = [H+(aq)] [OH¯(aq)] mol 2 dm-6 = 1 x 10 -14 mol 2 dm-6 (at 25°C)

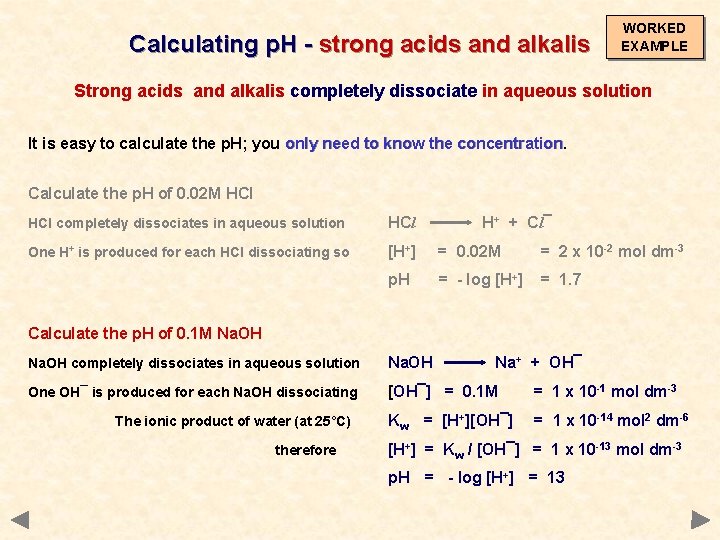
Calculating p. H - strong acids and alkalis WORKED EXAMPLE Strong acids and alkalis completely dissociate in aqueous solution It is easy to calculate the p. H; you only need to know the concentration Calculate the p. H of 0. 02 M HCl completely dissociates in aqueous solution HCl H+ + Cl¯ One H+ is produced for each HCl dissociating so [H+] = 0. 02 M = 2 x 10 -2 mol dm-3 p. H = - log [H+] = 1. 7

Calculating p. H - strong acids and alkalis WORKED EXAMPLE Strong acids and alkalis completely dissociate in aqueous solution It is easy to calculate the p. H; you only need to know the concentration Calculate the p. H of 0. 02 M HCl completely dissociates in aqueous solution HCl H+ + Cl¯ One H+ is produced for each HCl dissociating so [H+] = 0. 02 M = 2 x 10 -2 mol dm-3 p. H = - log [H+] = 1. 7 Calculate the p. H of 0. 1 M Na. OH completely dissociates in aqueous solution Na. OH One OH¯ is produced for each Na. OH dissociating [OH¯] = 0. 1 M = 1 x 10 -1 mol dm-3 Kw = [H+][OH¯] = 1 x 10 -14 mol 2 dm-6 The ionic product of water (at 25°C) therefore Na+ + OH¯ [H+] = Kw / [OH¯] = 1 x 10 -13 mol dm-3 p. H = - log [H+] = 13

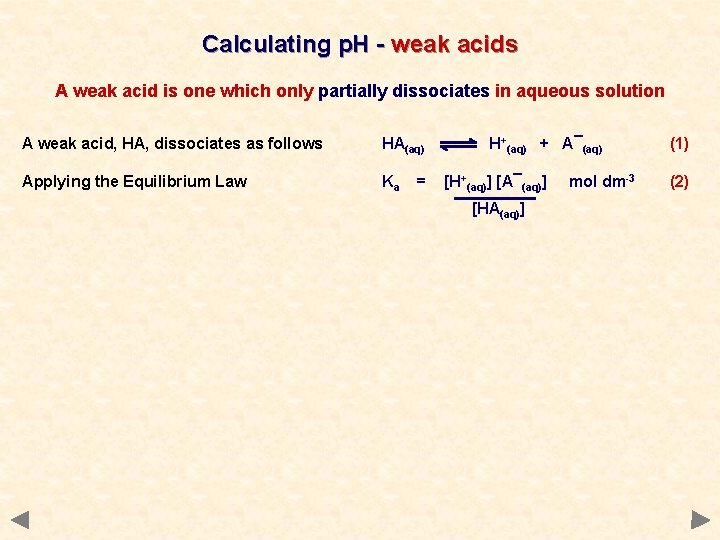
Calculating p. H - weak acids A weak acid is one which only partially dissociates in aqueous solution A weak acid, HA, dissociates as follows HA(aq) H+(aq) + A¯(aq) (1)

Calculating p. H - weak acids A weak acid is one which only partially dissociates in aqueous solution A weak acid, HA, dissociates as follows HA(aq) Applying the Equilibrium Law Ka = H+(aq) + A¯(aq) [H+(aq)] [A¯(aq)] [HA(aq)] mol dm-3 (1) (2)

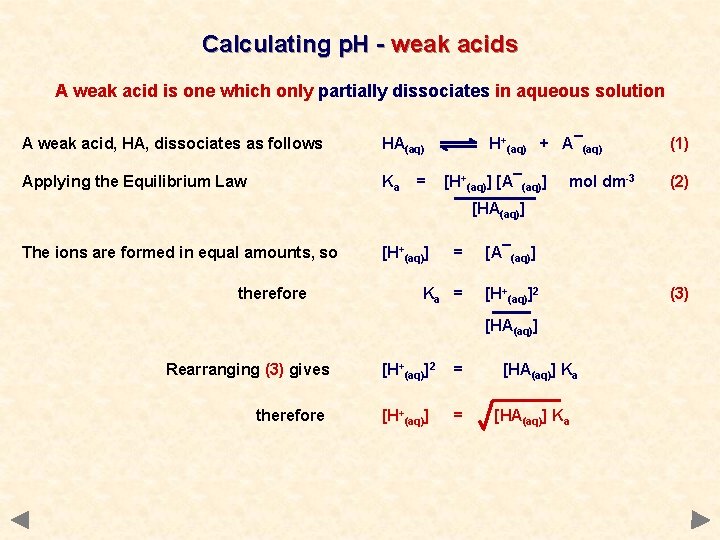
Calculating p. H - weak acids A weak acid is one which only partially dissociates in aqueous solution A weak acid, HA, dissociates as follows HA(aq) Applying the Equilibrium Law Ka = H+(aq) + A¯(aq) [H+(aq)] [A¯(aq)] mol dm-3 (1) (2) [HA(aq)] The ions are formed in equal amounts, so therefore [H+(aq)] = [A¯(aq)] Ka = [H+(aq)]2 [HA(aq)] (3)

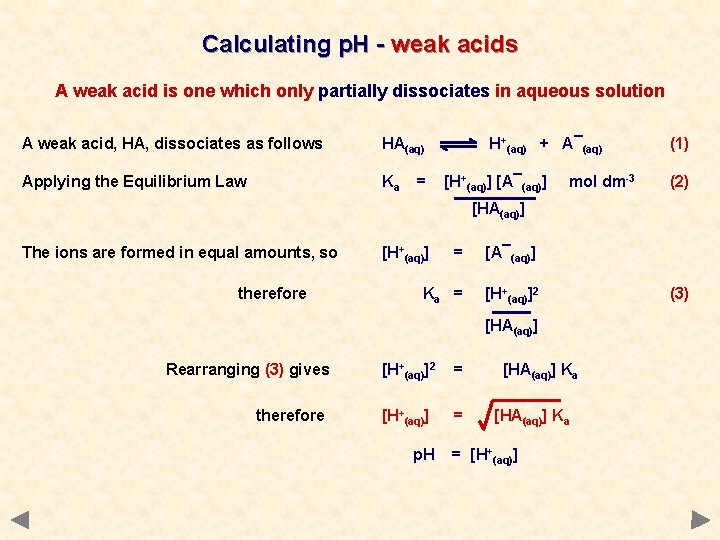
Calculating p. H - weak acids A weak acid is one which only partially dissociates in aqueous solution A weak acid, HA, dissociates as follows HA(aq) Applying the Equilibrium Law Ka = H+(aq) + A¯(aq) [H+(aq)] [A¯(aq)] mol dm-3 (1) (2) [HA(aq)] The ions are formed in equal amounts, so therefore [H+(aq)] = [A¯(aq)] Ka = [H+(aq)]2 [HA(aq)] Rearranging (3) gives therefore [H+(aq)]2 = [H+(aq)] = [HA(aq)] Ka (3)

Calculating p. H - weak acids A weak acid is one which only partially dissociates in aqueous solution A weak acid, HA, dissociates as follows HA(aq) Applying the Equilibrium Law Ka = H+(aq) + A¯(aq) [H+(aq)] [A¯(aq)] mol dm-3 (1) (2) [HA(aq)] The ions are formed in equal amounts, so therefore [H+(aq)] = [A¯(aq)] Ka = [H+(aq)]2 [HA(aq)] Rearranging (3) gives therefore [H+(aq)]2 = [H+(aq)] = p. H [HA(aq)] Ka = [H+(aq)] (3)

Calculating p. H - weak acids A weak acid is one which only partially dissociates in aqueous solution A weak acid, HA, dissociates as follows HA(aq) Applying the Equilibrium Law Ka = H+(aq) + A¯(aq) [H+(aq)] [A¯(aq)] mol dm-3 (1) (2) [HA(aq)] The ions are formed in equal amounts, so therefore [H+(aq)] = [A¯(aq)] Ka = [H+(aq)]2 (3) [HA(aq)] Rearranging (3) gives therefore [H+(aq)]2 = [H+(aq)] = p. H ASSUMPTION [HA(aq)] Ka = [H+(aq)] HA is a weak acid so it will not have dissociated very much. You can assume that its equilibrium concentration is approximately that of the original concentration.

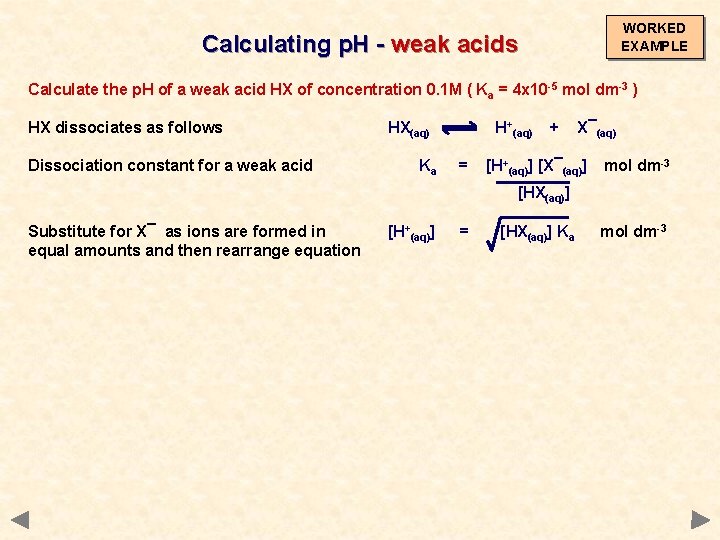
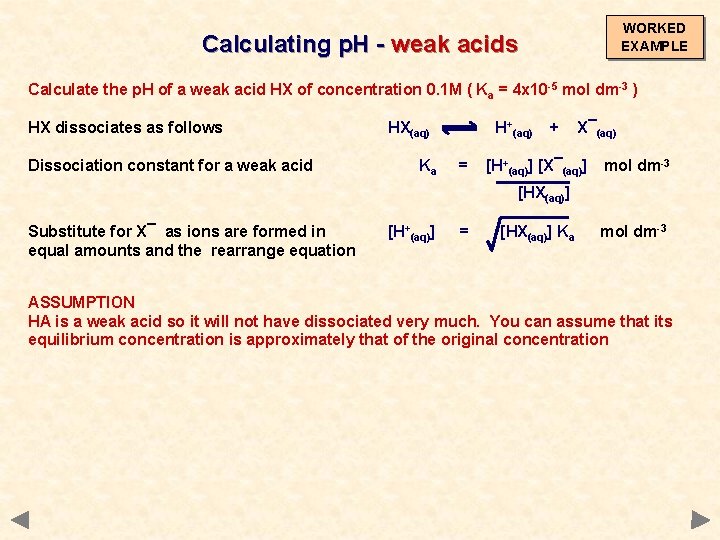
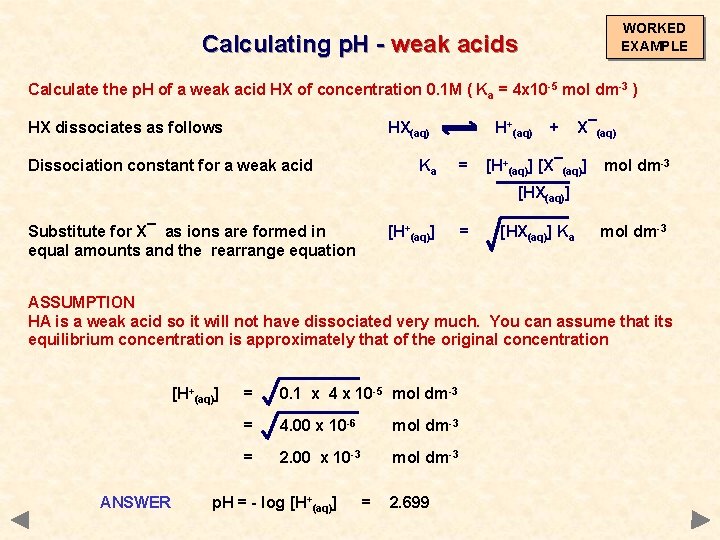
WORKED EXAMPLE Calculating p. H - weak acids Calculate the p. H of a weak acid HX of concentration 0. 1 M ( Ka = 4 x 10 -5 mol dm-3 ) HX dissociates as follows HX(aq) H+(aq) + X¯(aq)

WORKED EXAMPLE Calculating p. H - weak acids Calculate the p. H of a weak acid HX of concentration 0. 1 M ( Ka = 4 x 10 -5 mol dm-3 ) HX dissociates as follows Dissociation constant for a weak acid HX(aq) Ka H+(aq) = + X¯(aq) [H+(aq)] [X¯(aq)] [HX(aq)] mol dm-3

WORKED EXAMPLE Calculating p. H - weak acids Calculate the p. H of a weak acid HX of concentration 0. 1 M ( Ka = 4 x 10 -5 mol dm-3 ) HX dissociates as follows Dissociation constant for a weak acid HX(aq) Ka H+(aq) = + X¯(aq) [H+(aq)] [X¯(aq)] mol dm-3 [HX(aq)] Substitute for X¯ as ions are formed in equal amounts and then rearrange equation [H+(aq)] = [HX(aq)] Ka mol dm-3

WORKED EXAMPLE Calculating p. H - weak acids Calculate the p. H of a weak acid HX of concentration 0. 1 M ( Ka = 4 x 10 -5 mol dm-3 ) HX dissociates as follows Dissociation constant for a weak acid HX(aq) Ka H+(aq) = + X¯(aq) [H+(aq)] [X¯(aq)] mol dm-3 [HX(aq)] Substitute for X¯ as ions are formed in equal amounts and the rearrange equation [H+(aq)] = [HX(aq)] Ka mol dm-3 ASSUMPTION HA is a weak acid so it will not have dissociated very much. You can assume that its equilibrium concentration is approximately that of the original concentration

WORKED EXAMPLE Calculating p. H - weak acids Calculate the p. H of a weak acid HX of concentration 0. 1 M ( Ka = 4 x 10 -5 mol dm-3 ) HX dissociates as follows HX(aq) Dissociation constant for a weak acid Ka H+(aq) = + X¯(aq) [H+(aq)] [X¯(aq)] mol dm-3 [HX(aq)] Substitute for X¯ as ions are formed in equal amounts and the rearrange equation [H+(aq)] = [HX(aq)] Ka mol dm-3 ASSUMPTION HA is a weak acid so it will not have dissociated very much. You can assume that its equilibrium concentration is approximately that of the original concentration [H+(aq)] ANSWER = 0. 1 x 4 x 10 -5 mol dm-3 = 4. 00 x 10 -6 mol dm-3 = 2. 00 x 10 -3 mol dm-3 p. H = - log [H+(aq)] = 2. 699
 Types of connections in steel structures
Types of connections in steel structures Noble samn
Noble samn Fascia clavipectoral
Fascia clavipectoral Electronic table of contents
Electronic table of contents Civics interactive notebook
Civics interactive notebook Company profile table of contents
Company profile table of contents Contents of the middle mediastinum
Contents of the middle mediastinum Ip contents
Ip contents Contents of a dead man's pocket conflict
Contents of a dead man's pocket conflict Spermatic cord covering
Spermatic cord covering Sas contents
Sas contents Table of contents error
Table of contents error N
N Suprasternal space of burns contents
Suprasternal space of burns contents Mediastinum blood supply
Mediastinum blood supply The vertebral region is to the scapular region
The vertebral region is to the scapular region I-need-a-few-pointers-98hpou5
I-need-a-few-pointers-98hpou5 Table of contents annex
Table of contents annex