Overview of Adaptive Treatment Regimes Sachiko Miyahara Dr















- Slides: 40

Overview of Adaptive Treatment Regimes Sachiko Miyahara Dr. Abdus Wahed

Before starting the presentation… Adaptive Treatment Regimes ≠ Adaptive Experimental Design

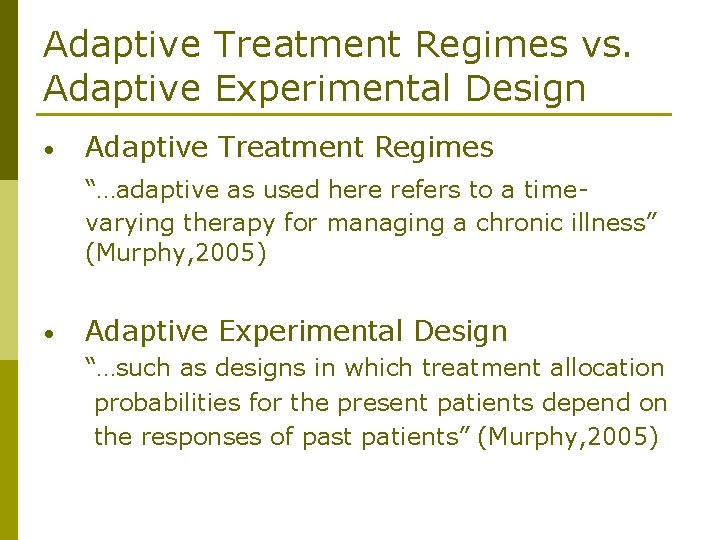
Adaptive Treatment Regimes vs. Adaptive Experimental Design • Adaptive Treatment Regimes “…adaptive as used here refers to a timevarying therapy for managing a chronic illness” (Murphy, 2005) • Adaptive Experimental Design “…such as designs in which treatment allocation probabilities for the present patients depend on the responses of past patients” (Murphy, 2005)

Outline 1. What is Adaptive Treatment Regime? -Definition -Example -Objective 2. How to decide the best regime? - 3 different study designs - Comparison of 3 designs 3. Trial Example (STAR*D) 4. Inference on Adaptive Treatment Regimes

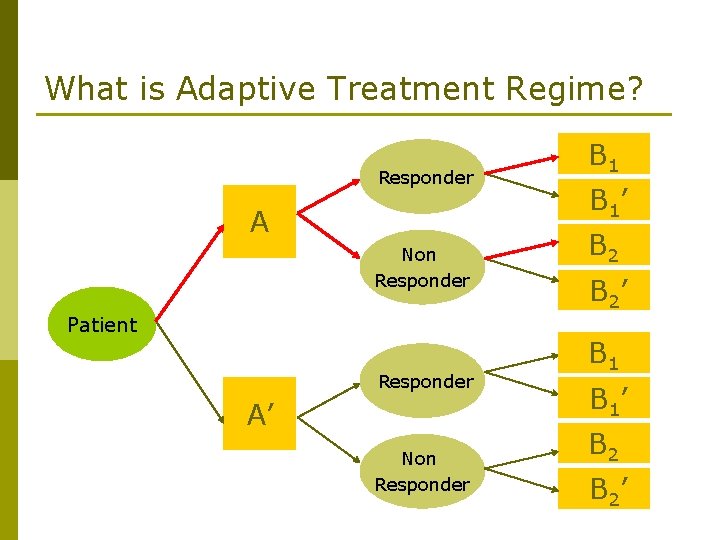
What is Adaptive Treatment Regime? Definition: a set of rule which select the best treatment option, which are made based on subjects’ condition up to that point.

What is Adaptive Treatment Regime? Responder A Non Responder Patient Responder A’ Non Responder B 1 B 1’ B 2 B 2’

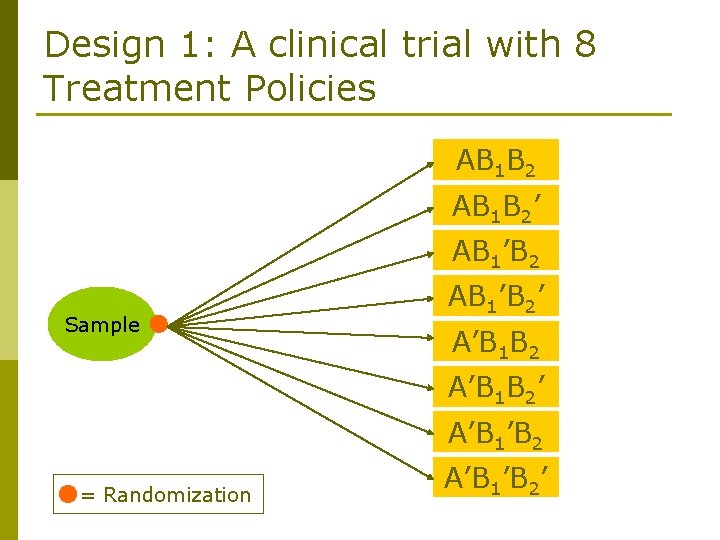
8 Possible Policies (1) Trt A followed by B 1 if response, else B 2 (AB 1 B 2) (2) Trt A followed by B 1 if response, else B 2’ (AB 1 B 2’) (3) Trt A followed by B 1’ if response, else B 2 (AB 1’B 2) (4) Trt A followed by B 1’ if response, else B 2’ (AB 1’B 2’) (5) Trt A’ followed by B 1 if response, else B 2 (A’B 1 B 2) (6) Trt A’ followed by B 1 if response, else B 2’ (A’B 1 B 2’) (7) Trt A’ followed by B 1’ if response, else B 2 (A’B 1’B 2) (8) Trt A’ followed by B 1’ if response, else B 2’ (A’B 1’B 2’)

What is the objective of the Adaptive Treatment Regimes? • Objective: To know which treatment strategy works the best, given a patient’s history.

What is the objective of the Adaptive Treatment Regime? A treatment naïve patient comes to a physician’s office. Questions: 1. What treatment strategy should the physician follow for that patient? 2. How should it be decided?

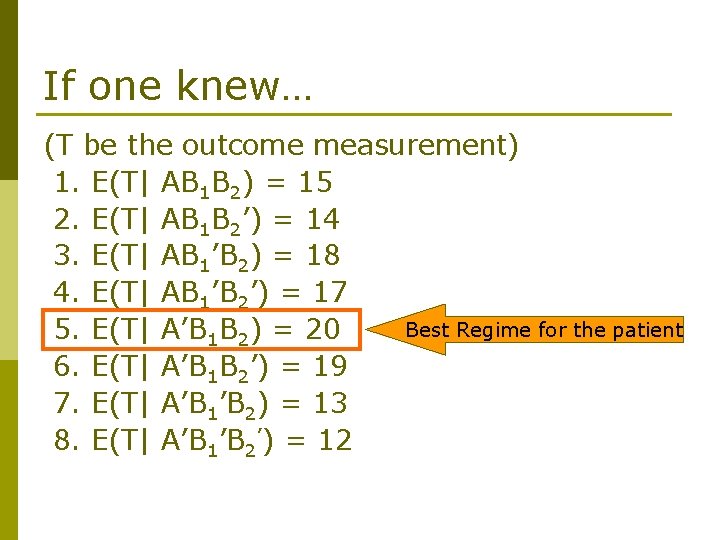
If one knew… (T be the outcome measurement) 1. E(T| AB 1 B 2) = 15 2. E(T| AB 1 B 2’) = 14 3. E(T| AB 1’B 2) = 18 4. E(T| AB 1’B 2’) = 17 Best Regime for the patient 5. E(T| A’B 1 B 2) = 20 6. E(T| A’B 1 B 2’) = 19 7. E(T| A’B 1’B 2) = 13 8. E(T| A’B 1’B 2’) = 12

In Reality… Problems: 1. E(T|. ) are not known (need to estimate) 2. How can one accurately and efficiently estimate E(T|. )?

How to estimate the expected outcome? Three study designs: 1. A clinical trial with 8 treatments 2. Combine existing trials 3. SMART (Sequential Multiple Assignment Randomized Trials)

Design 1: A clinical trial with 8 Treatment Policies AB 1 B 2’ AB 1’B 2 Sample AB 1’B 2’ A’B 1 B 2’ A’B 1’B 2 = Randomization A’B 1’B 2’

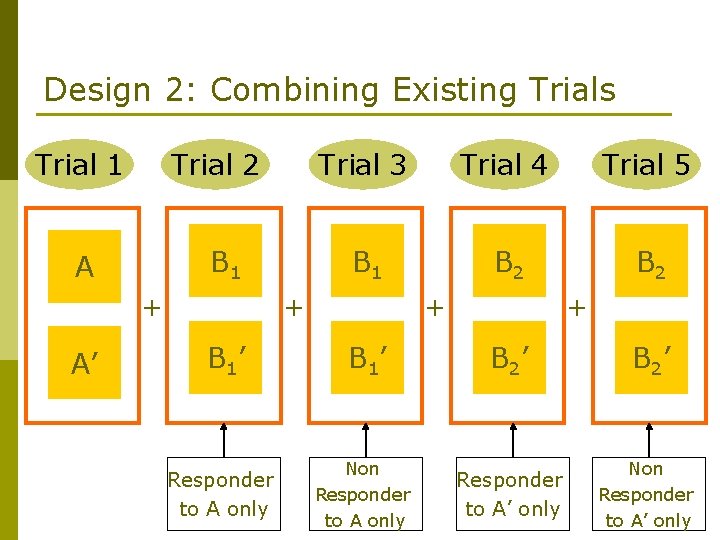
Design 2: Combining Existing Trials Trial 1 Trial 2 B 1 A + A’ Trial 3 Trial 4 Trial 5 B 1 B 2 + B 1’ Responder to A only + + B 1’ B 2’ Non Responder to A only Responder to A’ only Non Responder to A’ only

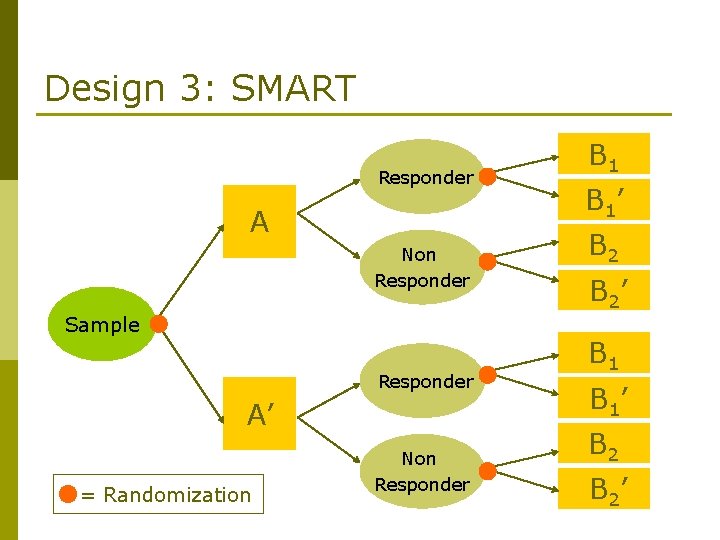
Design 3: SMART Sequential Multiple Assignment Randomized Trials (SMART) proposed by Dr. Murphy The SMART designs were adapted to: - Cancer (Thall 2000) - CATIE (Schneider 2001) – Alzheimer's Disease - STAR*D (Rush 2003) – Depression

Design 3: SMART Responder A Non Responder Sample Responder A’ = Randomization Non Responder B 1 B 1’ B 2 B 2’

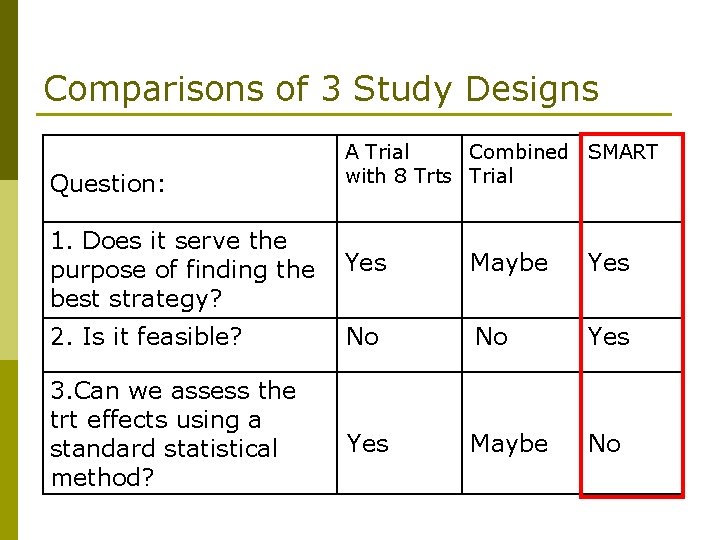
Comparisons of 3 Study Designs Question: A Trial Combined SMART with 8 Trts Trial 1. Does it serve the purpose of finding the best strategy? Yes Maybe Yes 2. Is it feasible? No No Yes 3. Can we assess the trt effects using a standard statistical method? Yes Maybe No

Sequenced Treatment Alternatives To Relieve Depression (STAR*D) 1. What is STAR*D? 2. The Study Design

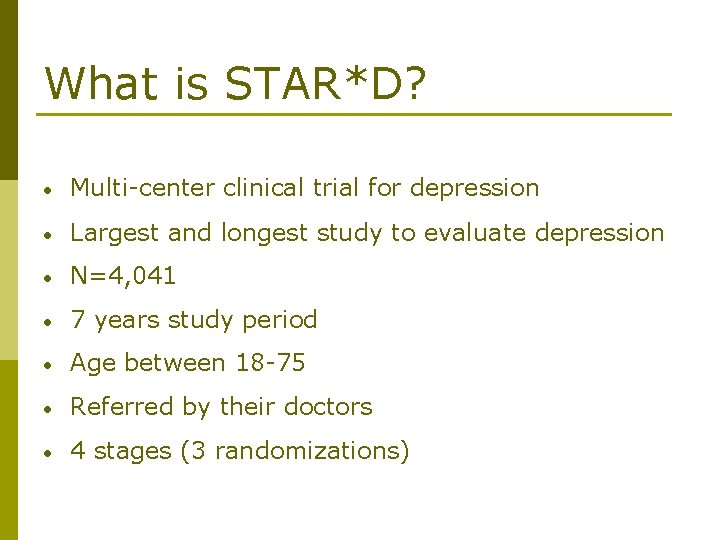
What is STAR*D? • Multi-center clinical trial for depression • Largest and longest study to evaluate depression • N=4, 041 • 7 years study period • Age between 18 -75 • Referred by their doctors • 4 stages (3 randomizations)

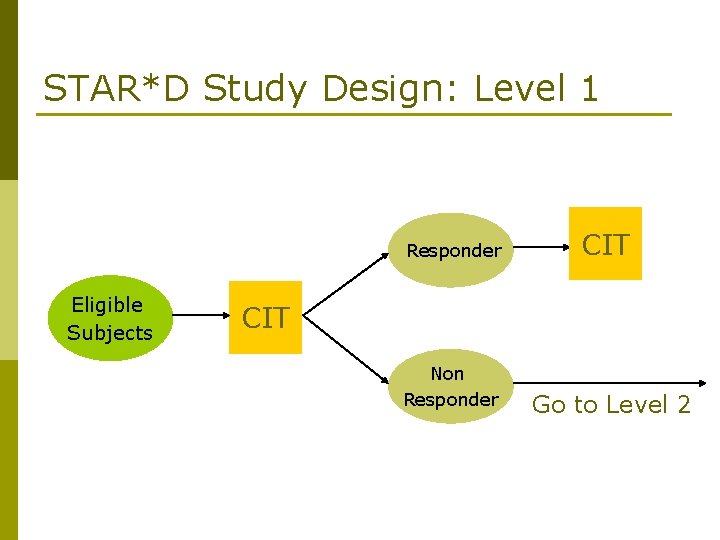
STAR*D Study Design: Level 1 Responder Eligible Subjects CIT Non Responder Go to Level 2

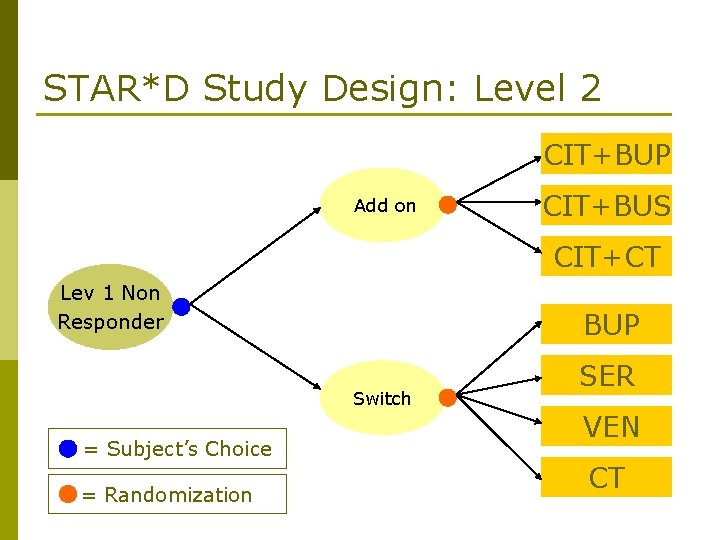
STAR*D Study Design: Level 2 CIT+BUP Add on CIT+BUS CIT+CT Lev 1 Non Responder BUP Switch = Subject’s Choice = Randomization SER VEN CT

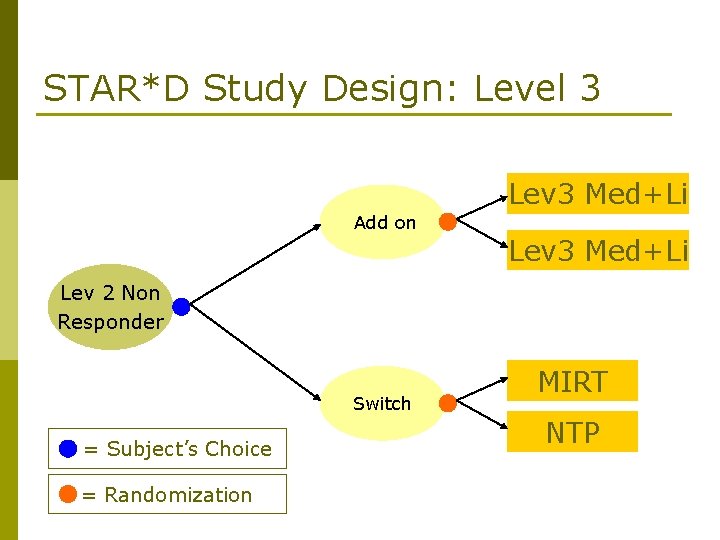
STAR*D Study Design: Level 3 Lev 3 Med+Li Add on Lev 3 Med+Li Lev 2 Non Responder Switch = Subject’s Choice = Randomization MIRT NTP

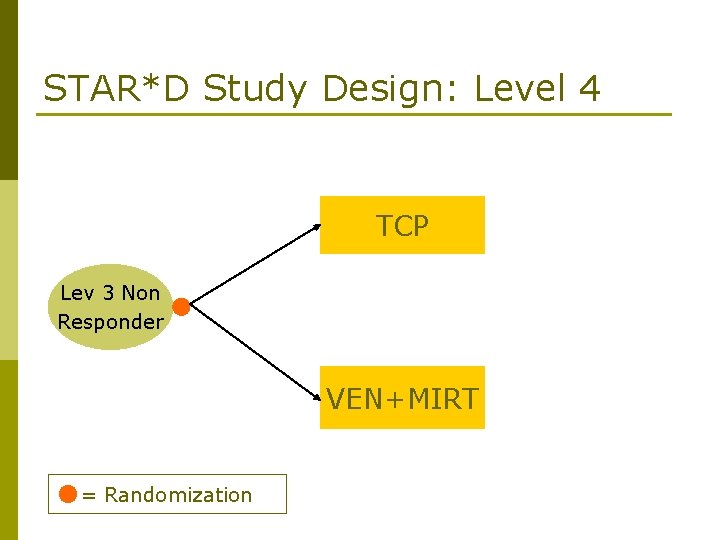
STAR*D Study Design: Level 4 TCP Lev 3 Non Responder VEN+MIRT = Randomization

Details on Inference from SMART • • Remember the goal is to estimate E(T|AB 1 B 2) First, how can we construct an unbiased estimator for E(T|AB 1 B 2)?

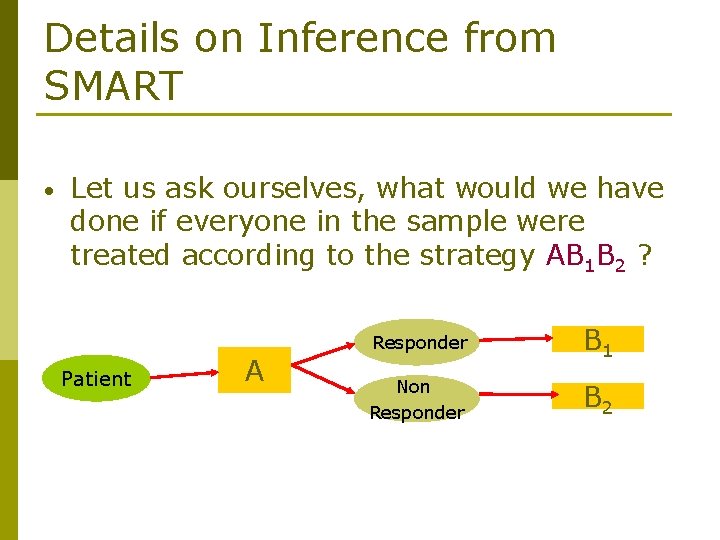
Details on Inference from SMART • Let us ask ourselves, what would we have done if everyone in the sample were treated according to the strategy AB 1 B 2 ? Patient A Responder B 1 Non Responder B 2

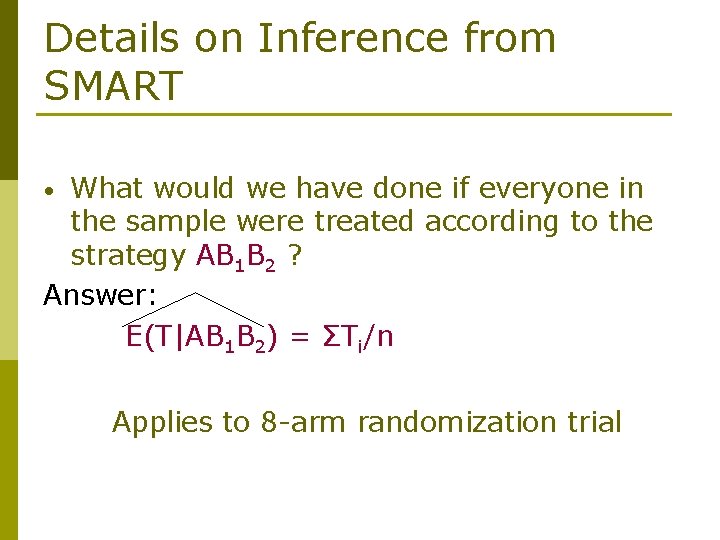
Details on Inference from SMART What would we have done if everyone in the sample were treated according to the strategy AB 1 B 2 ? Answer: E(T|AB 1 B 2) = ΣTi/n • Applies to 8 -arm randomization trial

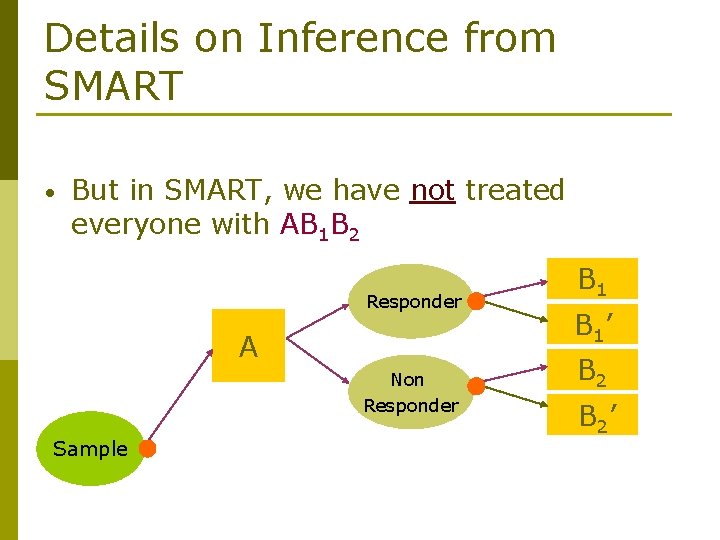
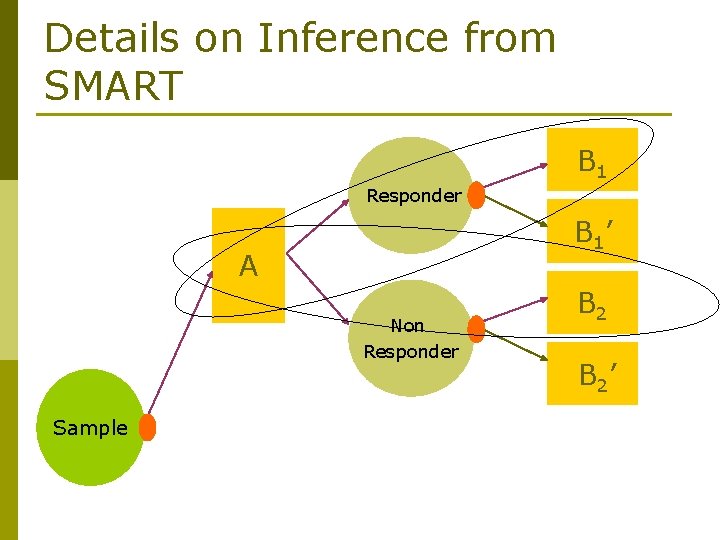
Details on Inference from SMART • But in SMART, we have not treated everyone with AB 1 B 2 Responder A Non Responder Sample B 1’ B 2’

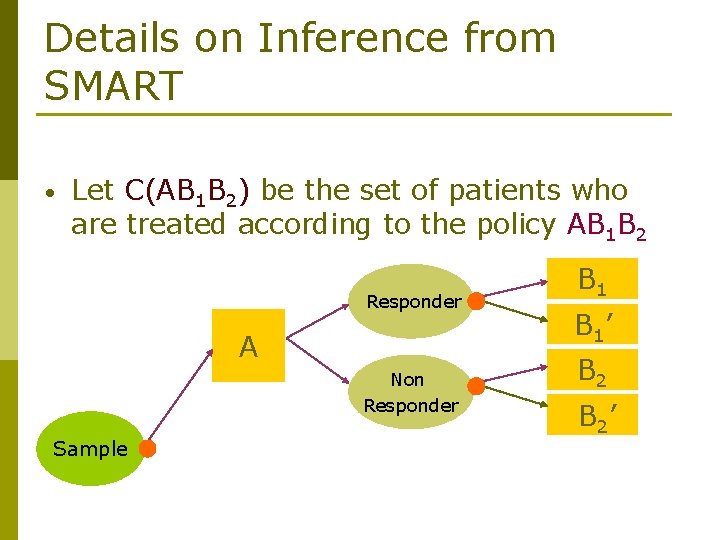
Details on Inference from SMART • Let C(AB 1 B 2) be the set of patients who are treated according to the policy AB 1 B 2 Responder A Non Responder Sample B 1’ B 2’

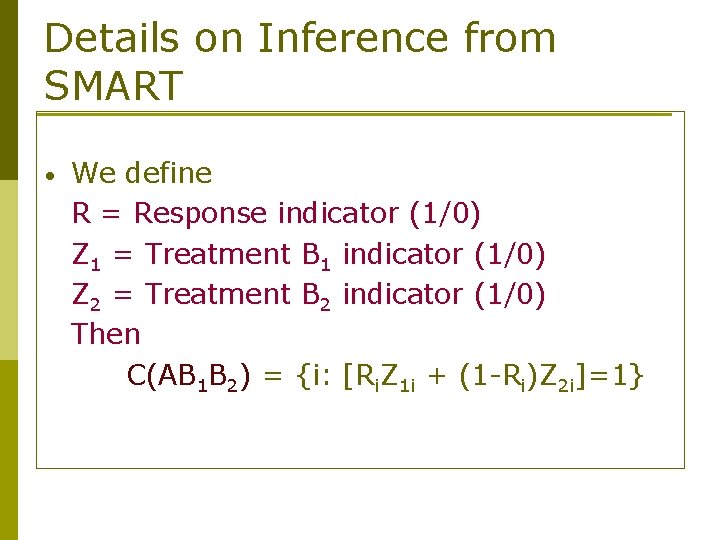
Details on Inference from SMART • We define R = Response indicator (1/0) Z 1 = Treatment B 1 indicator (1/0) Z 2 = Treatment B 2 indicator (1/0) Then C(AB 1 B 2) = {i: [Ri. Z 1 i + (1 -Ri)Z 2 i]=1}

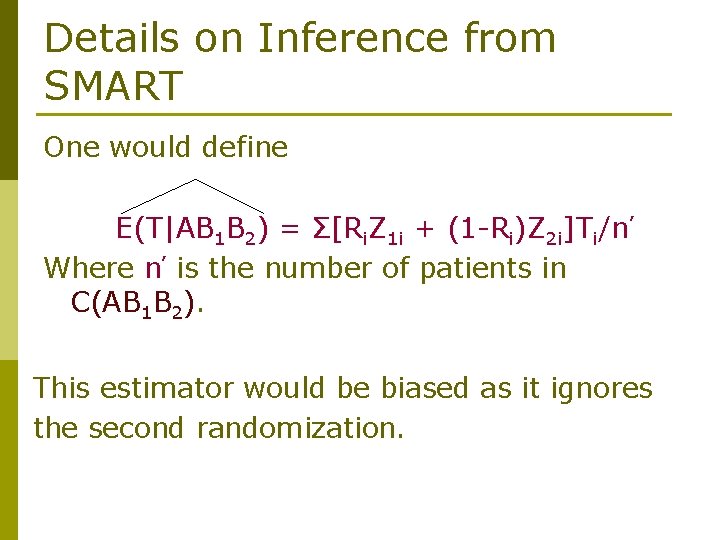
Details on Inference from SMART One would define E(T|AB 1 B 2) = Σ[Ri. Z 1 i + (1 -Ri)Z 2 i]Ti/n’ Where n’ is the number of patients in C(AB 1 B 2). This estimator would be biased as it ignores the second randomization.

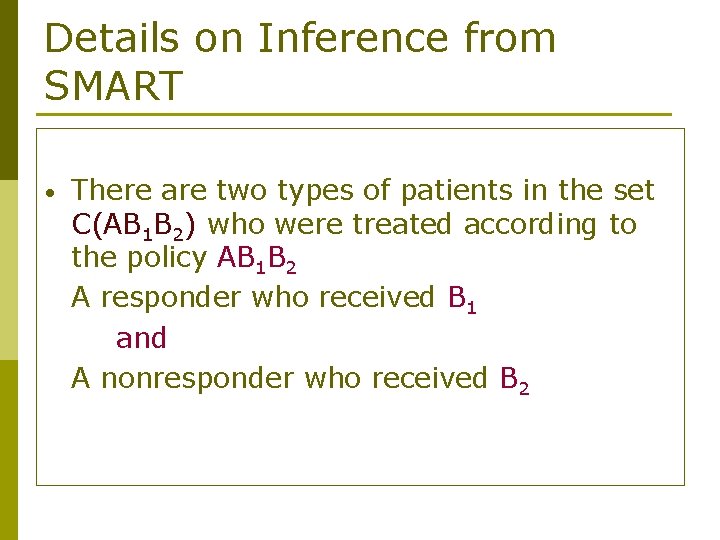
Details on Inference from SMART • There are two types of patients in the set C(AB 1 B 2) who were treated according to the policy AB 1 B 2 A responder who received B 1 and A nonresponder who received B 2

Details on Inference from SMART B 1 Responder B 1’ A Non Responder Sample B 2’

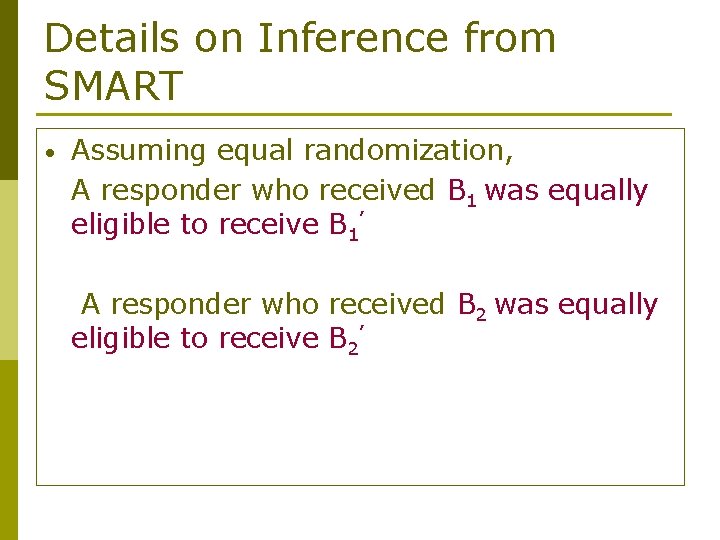
Details on Inference from SMART • Assuming equal randomization, A responder who received B 1 was equally eligible to receive B 1’ A responder who received B 2 was equally eligible to receive B 2’

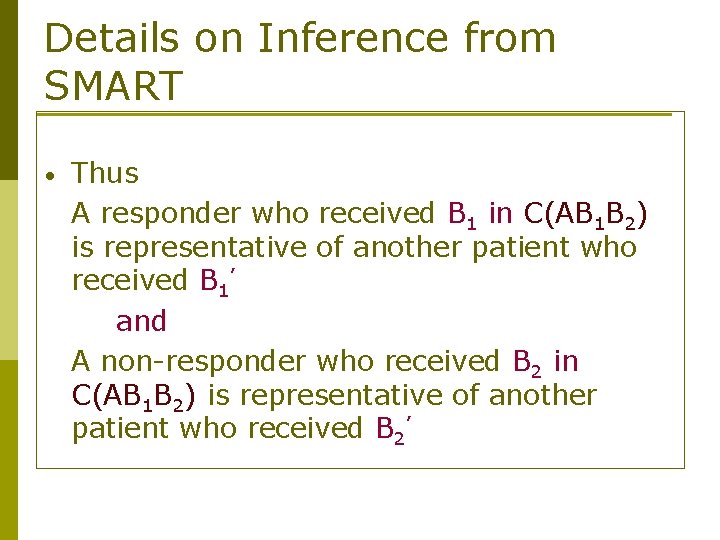
Details on Inference from SMART • Thus A responder who received B 1 in C(AB 1 B 2) is representative of another patient who received B 1’ and A non-responder who received B 2 in C(AB 1 B 2) is representative of another patient who received B 2’

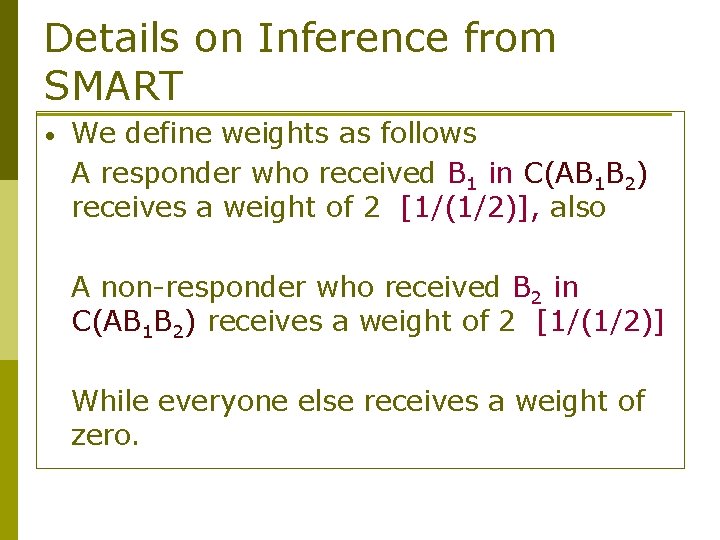
Details on Inference from SMART • We define weights as follows A responder who received B 1 in C(AB 1 B 2) receives a weight of 2 [1/(1/2)], also A non-responder who received B 2 in C(AB 1 B 2) receives a weight of 2 [1/(1/2)] While everyone else receives a weight of zero.

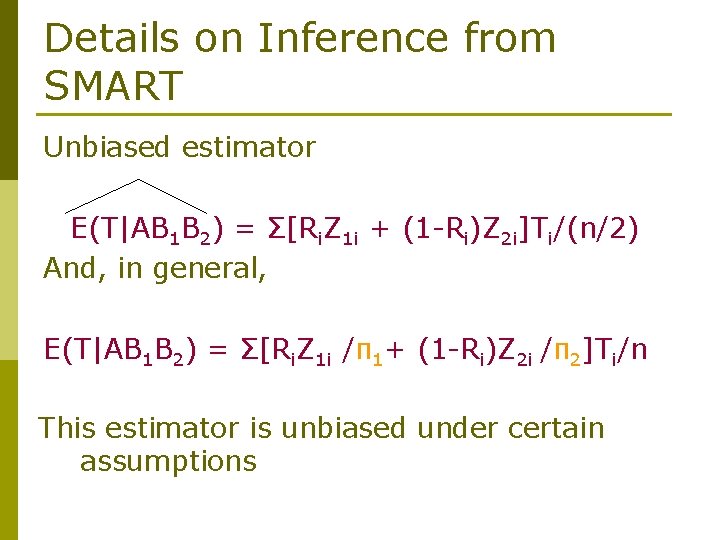
Details on Inference from SMART Unbiased estimator E(T|AB 1 B 2) = Σ[Ri. Z 1 i + (1 -Ri)Z 2 i]Ti/(n/2) And, in general, E(T|AB 1 B 2) = Σ[Ri. Z 1 i /π1+ (1 -Ri)Z 2 i /π2]Ti/n This estimator is unbiased under certain assumptions

Issues • Compare treatment strategies • • Wald test possible but needs to derive covariance between estimators (which may not be independent of each other) In survival analysis setting, how to derive formal tests to compare survival curves under different strategies • • Is log-rank test applicable? Can the proportional hazard model be applied here?

Issues • Efficiency issues • • How can one improve efficiency of the proposed estimator How to handle missing data (missing response information, censoring, etc. ) How to adjust for covariates when comparing treatment strategies And most importantly,

Issues • Is it possible to tailor the best treatment strategy decisions to individual characteristics? • For instance, could we one day hand over an algorithm to a nurse (not physician) which would provide decisions like “If the patient is a caucacian female, age 50 or over, have normal HGB levels, bla bla…the best strategy for maintaining her chronic disease would be……. . ”

ATSRG link http: //www. pitt. edu/~wahed/ATSRG/main. htm
 Régimes douaniers
Régimes douaniers The rise of dictatorial regimes
The rise of dictatorial regimes The seeds of totalitarian regimes are nurtured
The seeds of totalitarian regimes are nurtured The rise of dictatorial regimes lesson 2
The rise of dictatorial regimes lesson 2 Why were italian nationalists outraged after ww1
Why were italian nationalists outraged after ww1 Data analysis
Data analysis Sas customer intelligence
Sas customer intelligence Inert organizational culture
Inert organizational culture Whats adaptive radiation
Whats adaptive radiation Preserving statistical validity in adaptive data analysis
Preserving statistical validity in adaptive data analysis Adaptive cruise control
Adaptive cruise control Adaptive plasticity
Adaptive plasticity Self catheterization adaptive equipment
Self catheterization adaptive equipment Acm adaptive case management
Acm adaptive case management Deseasonalized demand formula
Deseasonalized demand formula Dolphins cheetah
Dolphins cheetah 5 adaptive features of camel
5 adaptive features of camel Adaptive maintenance in software engineering
Adaptive maintenance in software engineering Adaptive enrichment design
Adaptive enrichment design Adaptive defense system
Adaptive defense system Sitecore adaptive personalisation
Sitecore adaptive personalisation Vestigial
Vestigial H.264
H.264 Adaptive project life cycle
Adaptive project life cycle Adaptive leadership definition
Adaptive leadership definition Two level adaptive branch prediction
Two level adaptive branch prediction Adaptive and corrective maintenance
Adaptive and corrective maintenance Adaptive
Adaptive Mechanical isolatio
Mechanical isolatio Types of software changes
Types of software changes Domain adaptive faster rcnn
Domain adaptive faster rcnn Adaptive challenges examples
Adaptive challenges examples Linda r greene
Linda r greene Adaptive control
Adaptive control Fsa assessments org apm
Fsa assessments org apm Coévolution
Coévolution Decreased intracranial adaptive capacity
Decreased intracranial adaptive capacity Is adaptive radiation divergent evolution
Is adaptive radiation divergent evolution Adaptive products
Adaptive products Adaptive learning neural network
Adaptive learning neural network Adaptive challenge example
Adaptive challenge example