Adaptive Enrichment Designs for Confirmatory Clinical Trials Specifying














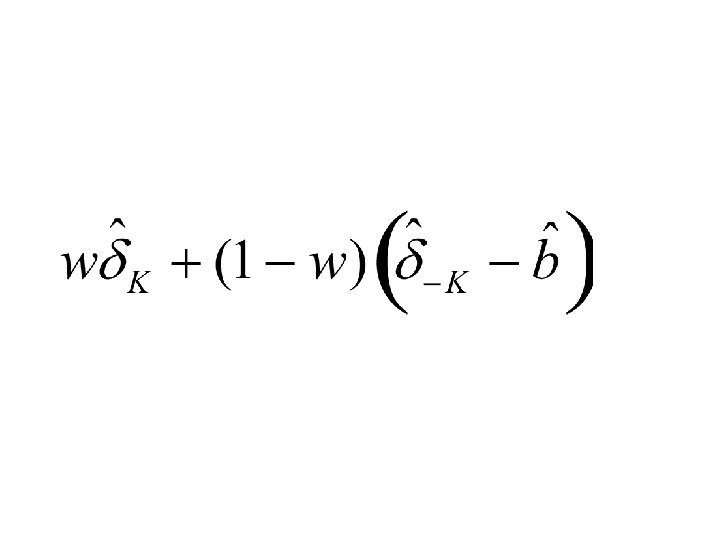



- Slides: 43

Adaptive Enrichment Designs for Confirmatory Clinical Trials Specifying the Intended Use Population and Estimating the Treatment Effect Richard Simon, D. Sc R Simon Consulting http: //rsimon. us Noah Simon, Ph. D. Department of Biostatistics University of Washington

Changed Strategies for Drug Development and Clinical Trial Design • Inter-patient variability of cancers of the same histologic type – Different oncogenic mutations that drive them – Different biologic behavior and response to treatment • Most new cancer treatments are very expensive & only work for a subset of patients with the disease

Need to Modify Standard Paradigm of Phase III Clinical Trials – Broad eligibility – Focus on ITT analysis

Standard Paradigm May Lead to • False negative studies • Positive studies with: – Large NNT – Small average treatment effects • High economic costs to society

Principle of New Paradigm • A phase III trial needs a primary analysis plan that preserves the study-wise type I error level, but that analysis need not be comparison of treatments for the allrandomized population

When The Drug Inhibits a Mutated Oncogene • The (targeted) enrichment design is the design of choice

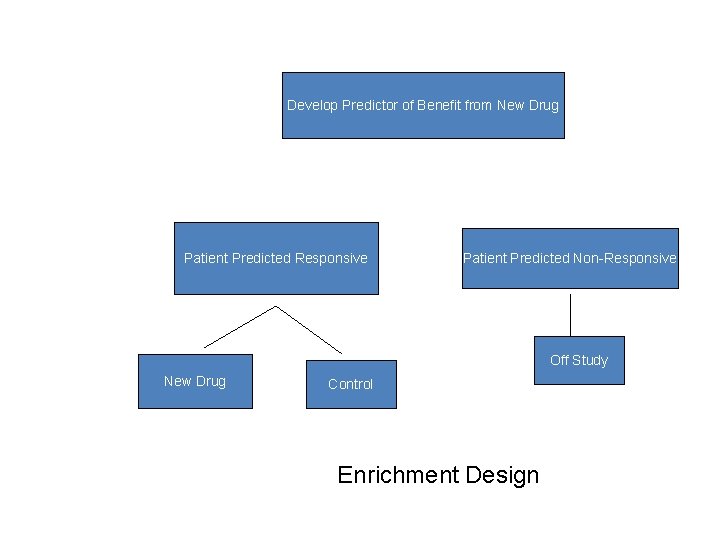
Using phase II data, develop predictor of response to new drug Develop Predictor of Benefit from New Drug Patient Predicted Responsive Patient Predicted Non-Responsive Off Study New Drug Control En Enrichment Design Enrichment design

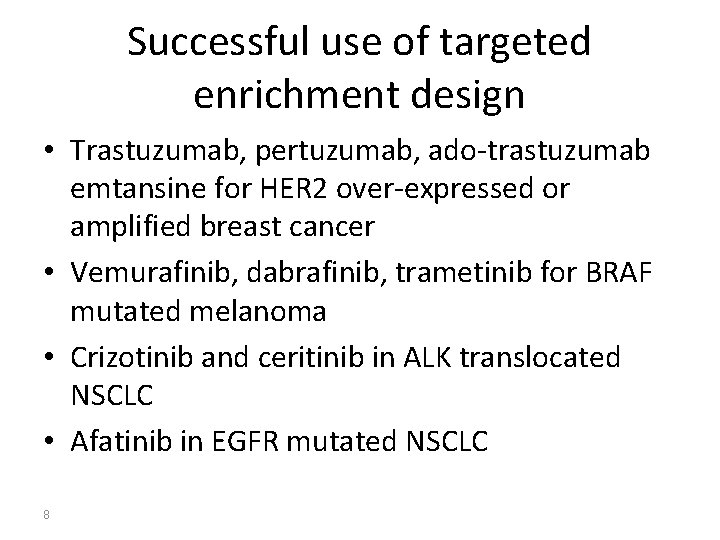
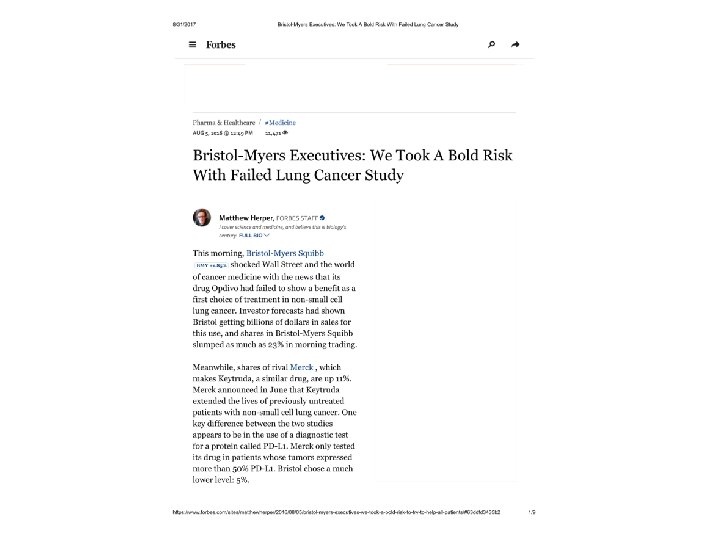
Successful use of targeted enrichment design • Trastuzumab, pertuzumab, ado-trastuzumab emtansine for HER 2 over-expressed or amplified breast cancer • Vemurafinib, dabrafinib, trametinib for BRAF mutated melanoma • Crizotinib and ceritinib in ALK translocated NSCLC • Afatinib in EGFR mutated NSCLC 8

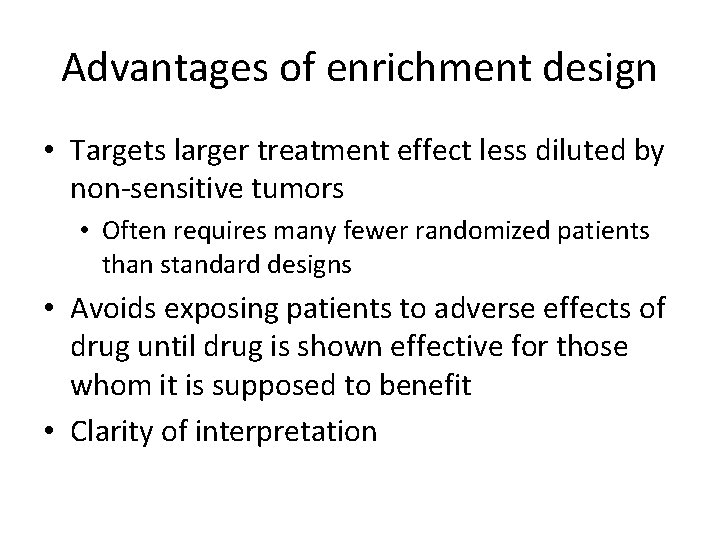
Advantages of enrichment design • Targets larger treatment effect less diluted by non-sensitive tumors • Often requires many fewer randomized patients than standard designs • Avoids exposing patients to adverse effects of drug until drug is shown effective for those whom it is supposed to benefit • Clarity of interpretation

• Cancer biology is complex and it is not always possible to have the right single predictive classifier identified with an appropriate cutpoint by the time the phase III trial of a new drug is ready to start accrual




“Adaptive keep your job design”

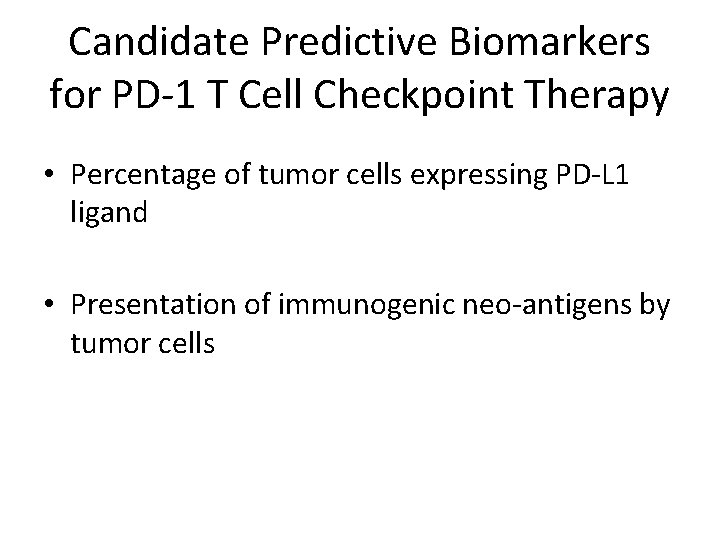
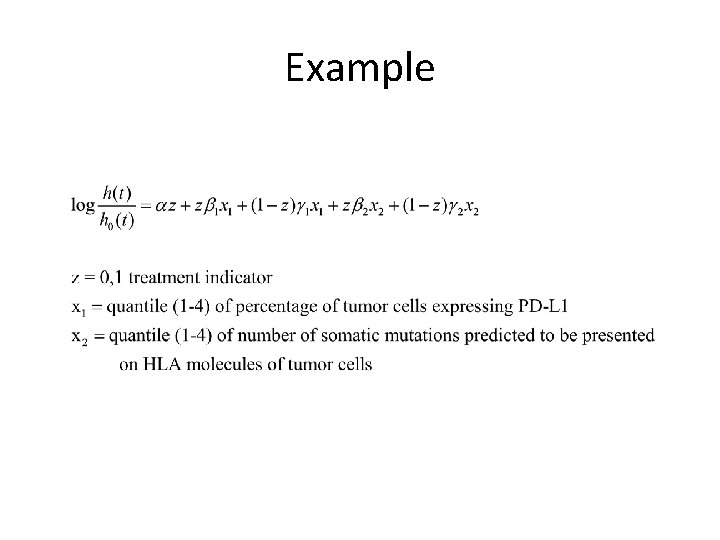
Candidate Predictive Biomarkers for PD-1 T Cell Checkpoint Therapy • Percentage of tumor cells expressing PD-L 1 ligand • Presentation of immunogenic neo-antigens by tumor cells

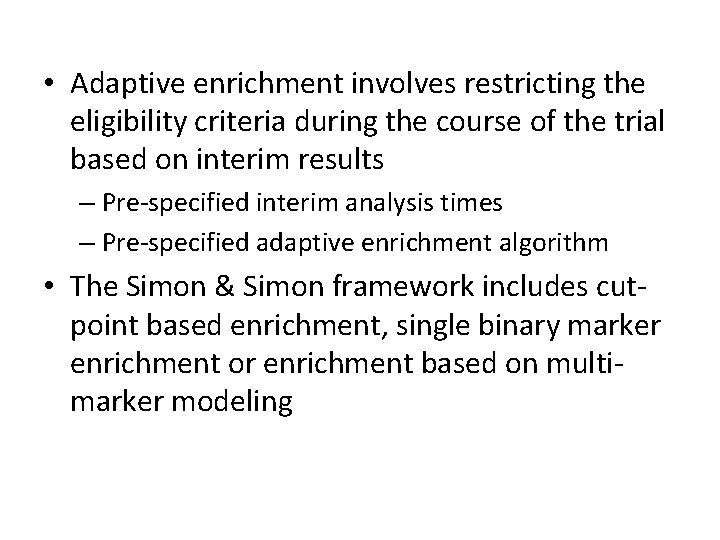
• Adaptive enrichment involves restricting the eligibility criteria during the course of the trial based on interim results – Pre-specified interim analysis times – Pre-specified adaptive enrichment algorithm • The Simon & Simon framework includes cutpoint based enrichment, single binary marker enrichment or enrichment based on multimarker modeling

Example

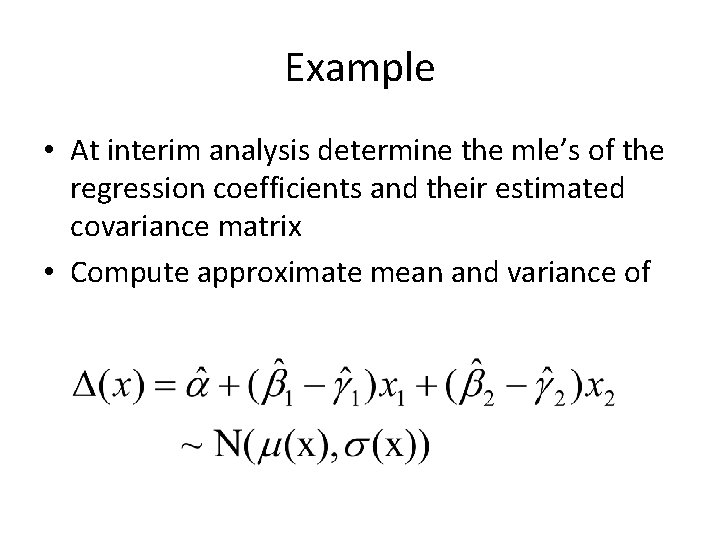
Example • At interim analysis determine the mle’s of the regression coefficients and their estimated covariance matrix • Compute approximate mean and variance of


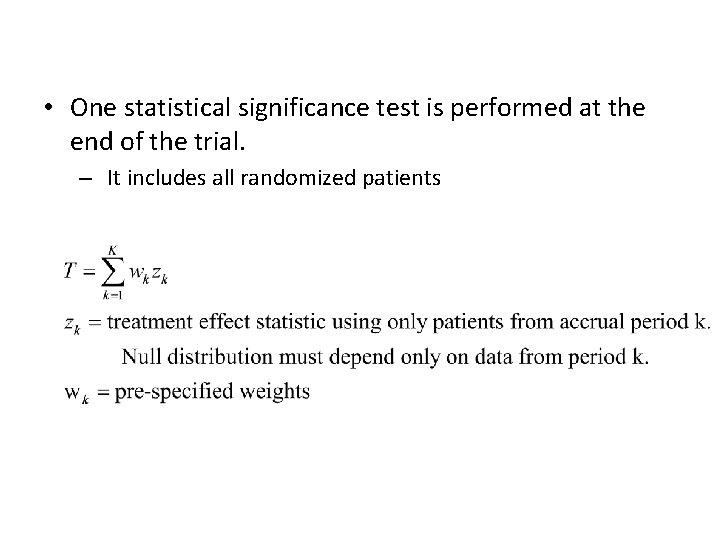
• One statistical significance test is performed at the end of the trial. – It includes all randomized patients

• Tests strong null that the treatments are equivalent for all patients • Fixed sample size regardless of changes in eligibility – Except for early termination for futility • Significance test preserves the study-wise type I error even with time dependent and data dependent changes to covariate distributions

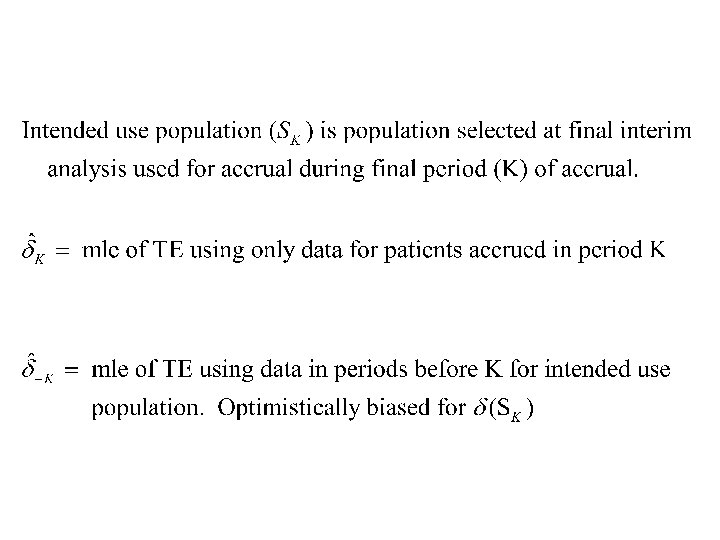
Survival Data • Use intermediate endpoint for adapting eligibility – PFS or response • zk=standardized log-rank statistic for patients accrued in period k but evaluated at final analysis time

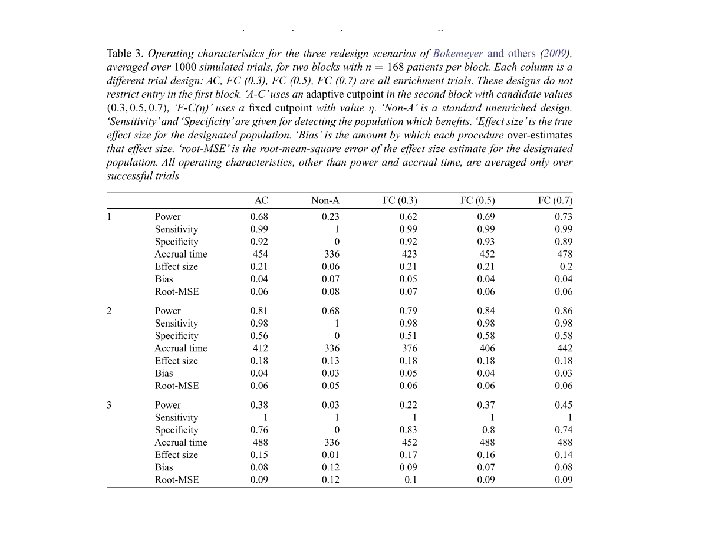
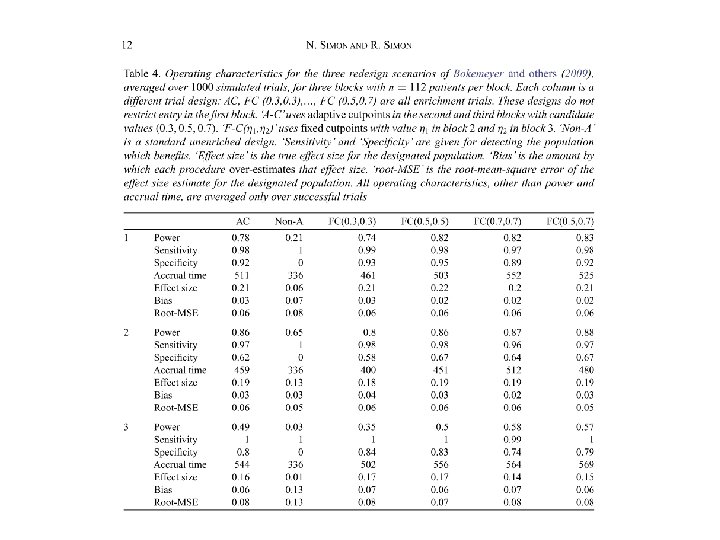
• Our simulations show that adaptive enrichment can substantially increase the statistical power with adaptive threshold determination and multi-biomarker modeling

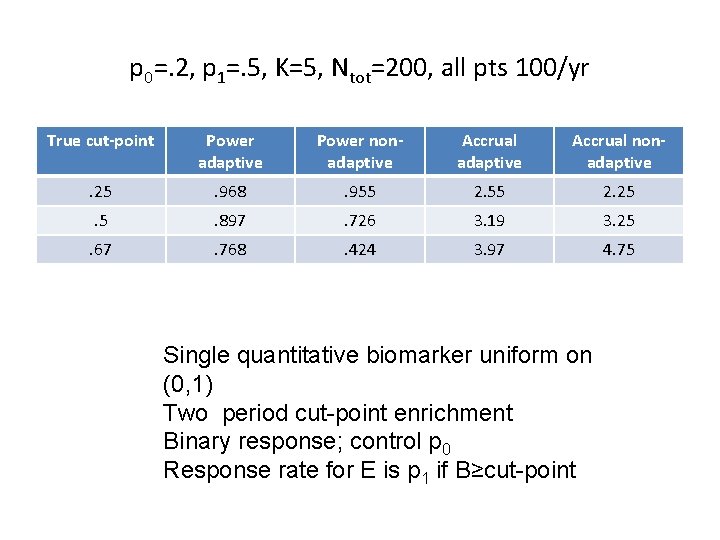
p 0=. 2, p 1=. 5, K=5, Ntot=200, all pts 100/yr True cut-point Power adaptive Power nonadaptive Accrual nonadaptive . 25 . 968 . 955 2. 25 . 897 . 726 3. 19 3. 25 . 67 . 768 . 424 3. 97 4. 75 Single quantitative biomarker uniform on (0, 1) Two period cut-point enrichment Binary response; control p 0 Response rate for E is p 1 if B≥cut-point


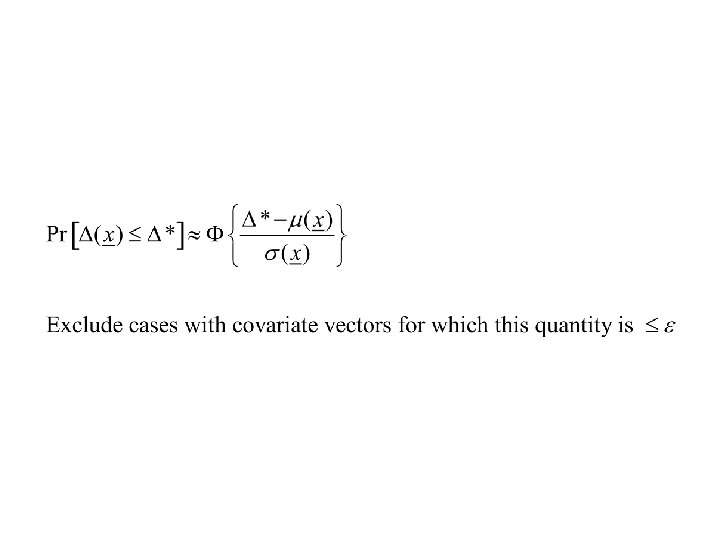
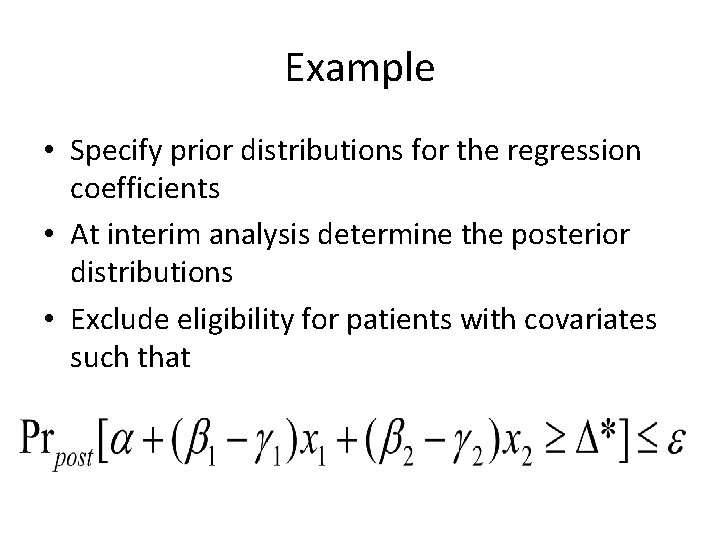
Example • Specify prior distributions for the regression coefficients • At interim analysis determine the posterior distributions • Exclude eligibility for patients with covariates such that








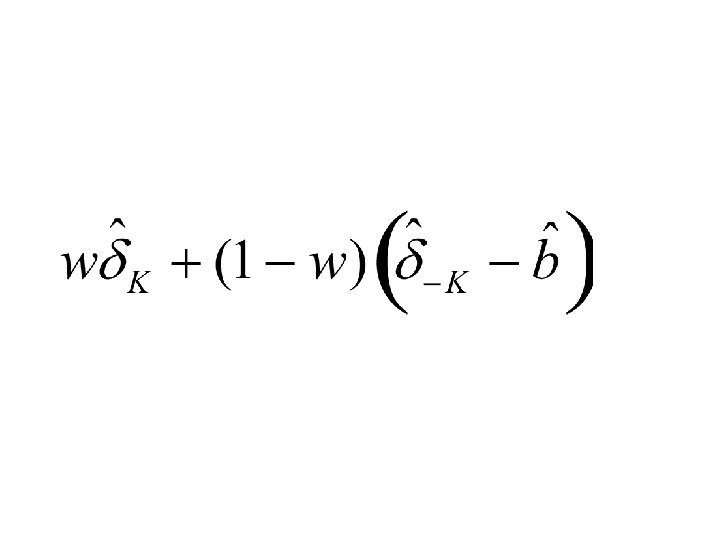

Description of Bootstrap De-biasing for a Twoperiod clinical trial • Outcome model as function of covariates is estimated using full dataset. • Bootstrap sample of outcomes for first period patients is generated from estimated outcome model • Eligibility restriction is determined for second period • Treatment effect in first period for patients eligible for second period is empirically determined – It is compared to treatment effect for those patients based on the model used to generate the data; the difference is an estimate of bias • Estimates of bias are averaged over multiple bootstrap samples

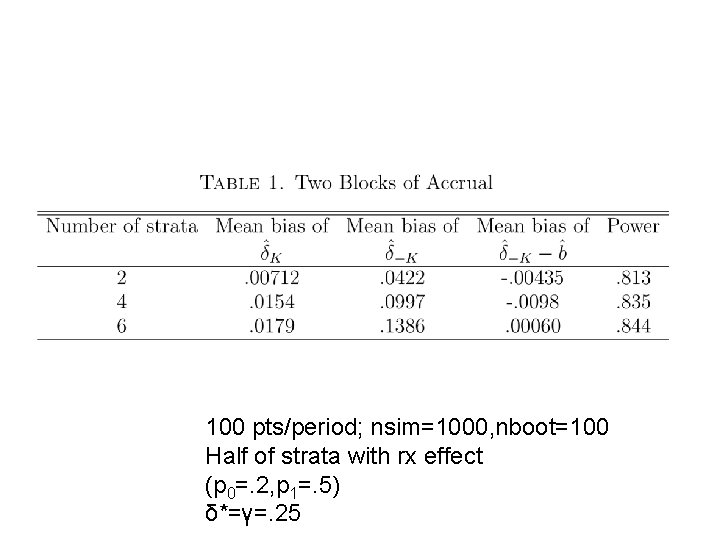
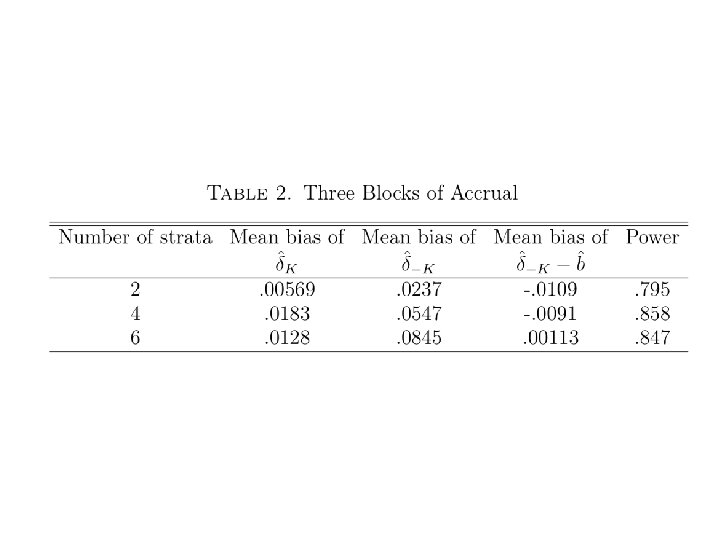
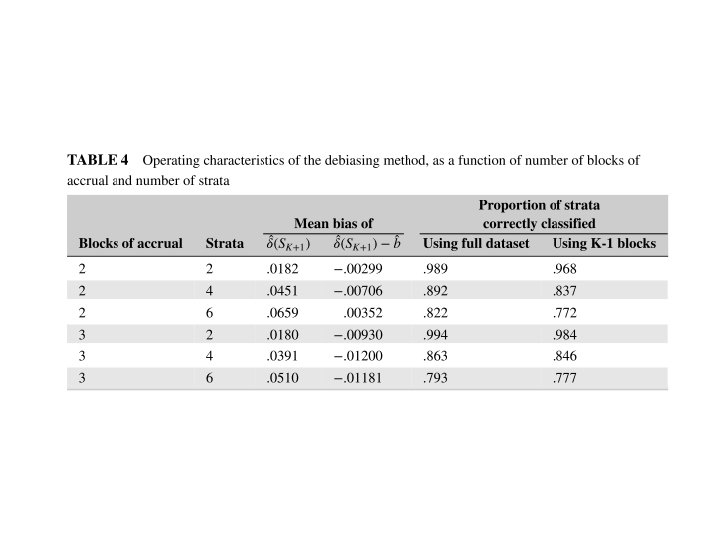
Simulation of J Disjoint Strata with Binary Response • True response probabilities for different strata are not modeled. They are estimated separately from the data. • p. T(j)=response probability of test rx for stratum j • p. C(j)=response probability of control for stratum j

Simulation of J Disjoint Strata with Binary Response • Restrict 2 nd period eligibility to stratum j if the likelihood of the stratum j period 1 data for the model p. T(j)=p. C(j)+δ* relative to the model p. T(j)=p. C(j) is < a threshold ϒ where δ* and ϒ are pre-specified.

100 pts/period; nsim=1000, nboot=100 Half of strata with rx effect (p 0=. 2, p 1=. 5) δ*=γ=. 25




Thank you
 Adaptive enrichment design
Adaptive enrichment design Prs registration
Prs registration Dhl atyrau
Dhl atyrau Phs human subjects and clinical trials information
Phs human subjects and clinical trials information Audits and inspections of clinical trials
Audits and inspections of clinical trials Role of statistician in clinical trials
Role of statistician in clinical trials Clinical trials.gov login
Clinical trials.gov login Mpn clinical trials
Mpn clinical trials Nida clinical trials network
Nida clinical trials network Clinical trials quality by design
Clinical trials quality by design Clinical hysteria salem witch trials
Clinical hysteria salem witch trials Mrc clinical trials unit
Mrc clinical trials unit Ivr iwr studies
Ivr iwr studies Hawk irb
Hawk irb Site initiation visit agenda
Site initiation visit agenda Professor claire harrison
Professor claire harrison Ohsu clinical trials office
Ohsu clinical trials office Randomization in statistics
Randomization in statistics York clinical trials unit
York clinical trials unit Clinical trials
Clinical trials Clinicaltrials.gov api
Clinicaltrials.gov api What is the purpose of research questions
What is the purpose of research questions Channel design paradigm
Channel design paradigm Cfa mplus syntax
Cfa mplus syntax Confirmation test for staphylococcus aureus
Confirmation test for staphylococcus aureus Luminol blood test
Luminol blood test What is exploratory research design
What is exploratory research design Pca stata
Pca stata Enrichment score
Enrichment score Semantic enrichment
Semantic enrichment D&b data enrichment
D&b data enrichment Enrichment clusters
Enrichment clusters Enrichment stage army
Enrichment stage army Job enrichment strategies
Job enrichment strategies Hockey diamante poem
Hockey diamante poem Catalog enrichment
Catalog enrichment Pengertian job enrichment
Pengertian job enrichment Enrichment culture technique
Enrichment culture technique Job description definition
Job description definition U of d mercy school of dentistry
U of d mercy school of dentistry David enrichment analysis
David enrichment analysis Job enrichment example
Job enrichment example Semantic content enrichment
Semantic content enrichment Job enrichment advantages
Job enrichment advantages