Orphan Products Natural History Grants Program The Office




















- Slides: 29

Orphan Products Natural History Grants Program The Office of Orphan Products Development Food and Drug Administration

Outline • • • • Background/ Natural History Definitions Objectives of OPD Natural History Grants Program Eligibility and Requirements Funding Levels Timeline How to Apply Review Process Merit Criteria Contents of Applications and Helpful Hints Responsibilities of Applicants & Grantees/Post Award Items FAQs How to Prepare Helpful Links Contacts

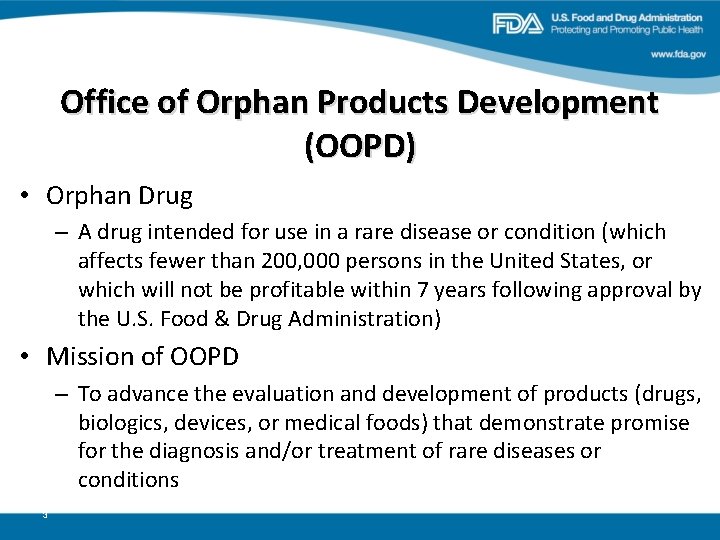
Office of Orphan Products Development (OOPD) • Orphan Drug – A drug intended for use in a rare disease or condition (which affects fewer than 200, 000 persons in the United States, or which will not be profitable within 7 years following approval by the U. S. Food & Drug Administration) • Mission of OOPD – To advance the evaluation and development of products (drugs, biologics, devices, or medical foods) that demonstrate promise for the diagnosis and/or treatment of rare diseases or conditions 3

Grant Programs in OOPD: 1. Clinical Trial Grants Program • To support the clinical development of products for use in rare diseases or conditions where no current therapy exists or where the proposed product will be superior to the existing therapy 2. Natural History Grants Program • To support studies that advance rare disease medical product development through characterization of the natural history of rare diseases/conditions, identification of genotypic and phenotypic subpopulations, and development and/or validation of clinical outcome measures, biomarkers and/or companion diagnostics 3. Pediatric Device Consortia Grant Program • To support the development of nonprofit consortia designed to stimulate projects which will promote pediatric device development

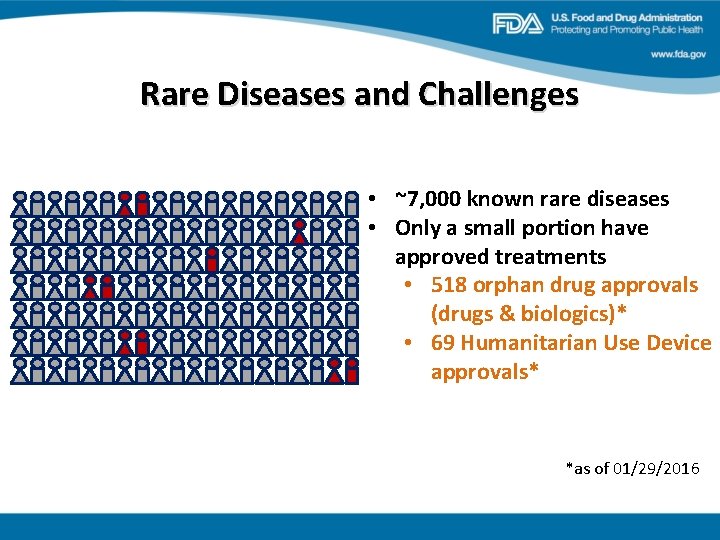
Rare Diseases and Challenges • ~7, 000 known rare diseases • Only a small portion have approved treatments • 518 orphan drug approvals (drugs & biologics)* • 69 Humanitarian Use Device approvals* *as of 01/29/2016

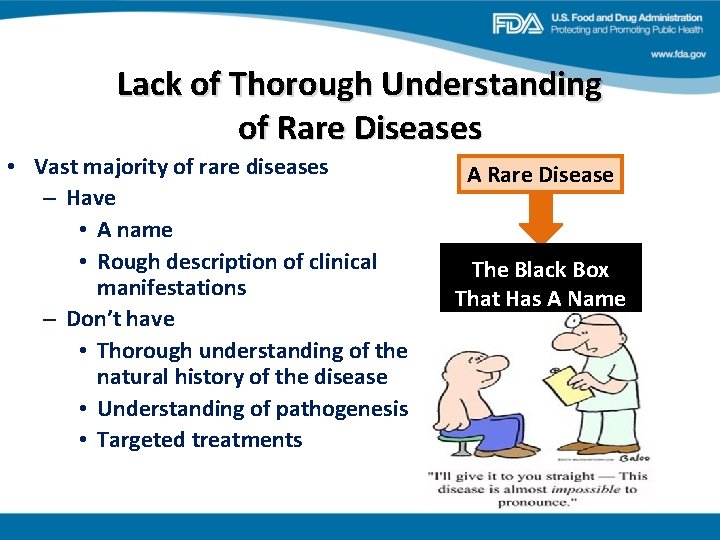
Lack of Thorough Understanding of Rare Diseases • Vast majority of rare diseases – Have • A name • Rough description of clinical manifestations – Don’t have • Thorough understanding of the natural history of the disease • Understanding of pathogenesis • Targeted treatments A Rare Disease The Black Box That Has A Name

The Essential First Step A thorough understanding of the natural history of a disease is the foundation of any treatment development program, especially for rare diseases!

Natural History Studies: Definitions • The Natural History of a disease is defined as the natural course of a disease from the time immediately prior to its inception, progressing through its pre-symptomatic phase and through the different clinical stages of the disease* • Natural History Studies track the course of disease over time, identifying demographic, genetic, environmental, and other variables that correlate with its development and outcomes* *Adapted from Posada de la Paz M, Groft S. Rare diseases epidemiology. Dordrecht: Springer; 2010.

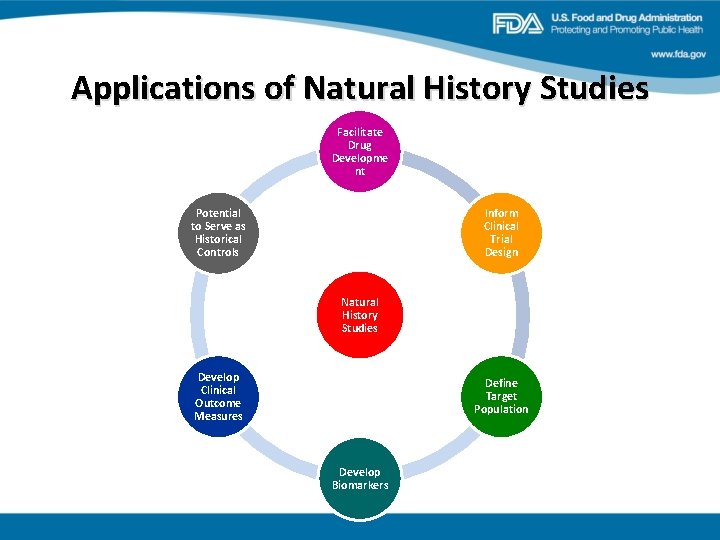
Applications of Natural History Studies Facilitate Drug Developme nt Potential to Serve as Historical Controls Inform Clinical Trial Design Natural History Studies Develop Clinical Outcome Measures Define Target Population Develop Biomarkers

Orphan Products Natural History Grants Program: Objectives • Targeted Natural History Studies – To support studies that advance rare disease medical product development through characterization of the natural history of rare diseases/conditions, including identification of genotypic and phenotypic subpopulations, and development and/or validation of clinical outcome measures, biomarkers and/or companion diagnostics – Ultimately, to assist market approval

Eligibility and Requirements • Eligibility: – Academic, industry and patient group sponsored research – Domestic or foreign, public or private, for-profit or nonprofit entities – Patients/patient advocacy groups, clinicians, researchers and industry partners • Requirements: – Study of an orphan disease or condition (Diseases that affect fewer than 200, 000 individuals in the US) – Prospective, retrospective or survey studies characterizing the natural history of a rare disease – A study must advance information towards a market approval – Human Subjects Assurance from OHRP (Office of Human Research Protections) “Federal-Wide Assurance or FWA” (www. hhs. gov/ohrp) – IRB approval

Funding Levels • Two Funding Levels – Prospective studies involving clinical examinations: Maximum of $400, 000 in total cost (direct and indirect costs) per year for up to five years – Retrospective analyses or Surveys: Maximum of $150, 000 in total cost (direct and indirect costs) per year for up to two years • ~$2 million to award 2 -5 grants in FY 2017 • Funding dependent on quality of application and availability of Federal funds

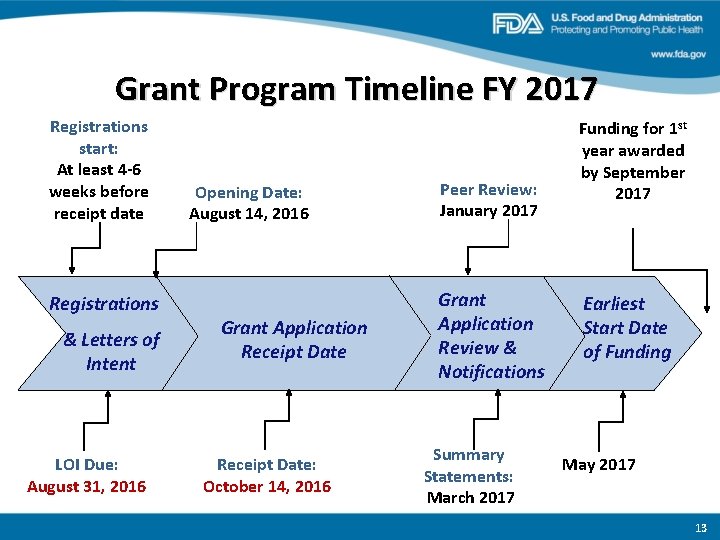
Grant Program Timeline FY 2017 Registrations start: At least 4 -6 weeks before receipt date Registrations & Letters of Intent LOI Due: August 31, 2016 Opening Date: August 14, 2016 Grant Application Receipt Date: October 14, 2016 Peer Review: January 2017 Grant Application Review & Notifications Summary Statements: March 2017 Funding for 1 st year awarded by September 2017 Earliest Start Date of Funding May 2017 13

How to Apply: Getting Started 1. Applicants should first review the detailed Request For Application(RFA) announcement. 2. Submit Letter of Intent (LOI) to FDA via email by August 31, 2016 3. Registrations Needed: Step 1: Obtain a Data Universal Number System (DUNS) number Step 2: Register with the System for Award Management (SAM) - A valid Taxpayer Identification Number (TIN) or Employer Identification Number (EIN) is necessary for SAM registration. Step 3: Register with and obtain Username & Password on Grants. gov Step 4: E-Business Point of Contact (EBiz POC) authorizes roles, which includes the Authorized Organization Representative (AOR) role on Grants. gov Step 5: Track Role Request Status Steps 1 through 5, in detail, can be found at http: //www. grants. gov/web/grants/applicants/organization-registration. html Step 6: Register with e. RA Commons

How to Apply: Submitting Applications 4. Grants. gov – Submit electronically through www. grants. gov • Application materials will open on Grants. gov ~ 60 days prior to the application receipt date) – Follow instructions under “Apply for Grants” • Search using RFA information (FD-16 -043) • Download copy of application package (SF-424 RR) and instructions – “Applicant Help” section provides User’s Guide, FAQs and other support • Complete offline • Upload and submit via grants. gov website • Track status of application via grants. gov Helpful hints available on OPD’s website (Note: Not all of the information in the NIH Application Guide will apply to the Orphan Products Natural History Grant applications. )

Review Process Initial Review by OOPD for Responsiveness; Notifications of Non-Responsiveness via email All responsive applications are individually reviewed and scored by independent ad hoc experts for technical merit Scoring based on Criteria in RFA Funding based on scores (1 -9 -> lower scores are better) Top scored applications will be selected for discussion by grant review panel Summary Statements Issued to all Applicants with review specifics Funding of top scored applications

Merit Criteria Ad hoc experts review and score applications based on the following 14 scientific and technical merit criteria: 1. The soundness of the rationale for the proposed study; 2. The quality and appropriateness of the study design, including the design of the monitoring and human subject protection plan; 3. If needed, the statistical justification for the number of patients chosen for the study, based on the proposed outcome measures, and the appropriateness of the statistical procedures for analysis of the results; 4. The adequacy of the evidence that the proposed number of eligible subjects can be recruited in the requested timeframe; 5. The qualifications of the investigator and support staff, and the resources available to them; 6. The adequacy of the justification for the request for financial support; 7. The adequacy of plans for complying with regulations for protection of human subjects and monitoring;

Merit Criteria (continued) 8. The ability of the applicant to complete the proposed study within its budget and within time limits stated in this Funding Opportunity Announcement (FOA); 9. Provision (and adequacy) of a plan to include patient/stakeholder input in study design and data elements of interest; 10. The soundness of rationale in relation to the understanding of the rare disease, description of prior and ongoing natural history studies and product development programs; 11. The adequacy of plans for ensuring data quality including, but not limited to standardized data entry and compliance to good clinical practice; 12. The adequacy of plans for an interim report for prospective longitudinal studies; 13. If applicable, the adequacy for sustainability plans for study continuation beyond the proposed funding period and for acquiring alternative/additional funding if needed; and 14. The adequacy for plans for data access and dissemination following completion of the study.

Content of Applications • • Project Summary/Abstract Project Narrative Facilities/Other Resources Senior/Key Personnel Profiles Budget Specific Aims Research Strategy – Background and Significance – Study Plan • • Protection of Human Subjects Inclusions/Exclusions Consortium/Contractual Arrangements Appendices – Protocol, Informed Consent form, Letters of Support, publications, data collection instruments, etc.

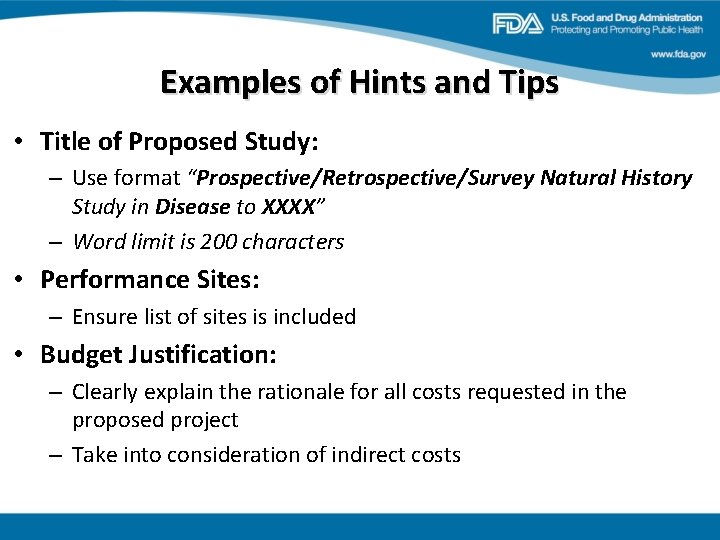
Examples of Hints and Tips • Title of Proposed Study: – Use format “Prospective/Retrospective/Survey Natural History Study in Disease to XXXX” – Word limit is 200 characters • Performance Sites: – Ensure list of sites is included • Budget Justification: – Clearly explain the rationale for all costs requested in the proposed project – Take into consideration of indirect costs

Examples of Hints and Tips Research Strategy Sections: – Background and Significance Section: • Provide and support prevalence estimate for the rare disease • Include the landscape of the disease including what are the current treatment options and standard of care options, what is being done in terms of assisting product development currently, what competing natural history studies are ongoing, and how this study will progress the existing knowledge • Document the importance, necessity and impact of the proposed study • Include information on Preliminary Studies • Explain how the proposed study will contribute to and support future and/or ongoing product development • If a retrospective study is proposed, provide justification of the necessity of the study vs a prospective study at this time – Study Plan Section: • Overall strategy, methodology, and analyses • Explain how patient/stakeholder input was included • Discuss challenges, potential problems, alternative strategies, and benchmarks for success anticipated to achieve the aims • Description of statistical analysis if applicable • If prospective study is proposed, provide interim analysis plans • Plans for data dissemination and data access • Sustainability of the study if applicable (e. g. , prospective studies, chronic diseases)

Following Review Panel • Applicants will receive summary statements and scores via email • For applications that receive a competitive score, applicants will need to: – Respond to Summary Statement Critiques – Complete OOPD Pre-Certification Form

Responsibilities of Future Grantees Once funded, Grantees will: – Set study goals with OOPD’S grant project officer (PO) – Maintain Regulatory requirements as necessary for your study (IRB, FWA, IND, Clinicaltrials. gov, GCP) – Submit Quarterly Progress Reports to the OOPD’s grant PO – Be encouraged to publish results and share data (see RFA) – Submit noncompeting continuation applications for following years with Progress Reports – Submit Final Report at the end of grant

How OOPD interacts with Grantees • Project Officer (PO) is assigned grant • Establishes study goals with grantee • Ensures regulatory requirements maintained (IRB approvals, FWA, etc. ) • Evaluates progress and makes recommendations for continued funding • If issues with study progress arise, PO will work with grantee

Future Years Support for Funded Grants • A grantee will submit noncompeting continuation requests yearly in order to receive future budget years of the grant. • Future years of noncompeting continuation support depends on: – Performance during the preceding year – Compliance with regulatory requirements – Availability of Federal funds

FAQs • • • Can patients in the study receive standard of care and/or emergent treatments? – Yes. Can I submit two separate applications (e. g. , retrospective and prospective studies)? – Yes; multiple applications are allowed as long as they are scientifically distinct. Do I have to submit a letter of intent? – LOIs are not required, but encouraged. Do you have a preference between multi- or single-center trials? – No; It is important to have justifications for design specifics and to ensure the study will be able to complete within time and budget. Can I have study sites outside of US? – Yes; foreign sites as well as foreign applicants are eligible under the RFA. Are certain diseases/populations being targeted for this RFA? – No; proposals will be scored based on technical merit criteria outlined in the RFA. Do I need to have a product being developed at this time? – No; future plans for potential product development should be included in the proposal. Do I need to submit the protocol in the application? – Yes; protocols and informed consent documents should be included as appendices. Do I need IRB approval at the time of the application? – No; IRB approvals will be needed at the time of funding. When is the next receipt date after 2016? – After October 14, 2016, the next anticipated receipt date is October 15, 2018. Additional FAQs are on OOPD’s website.

Plans for Success 1. 2. 3. 4. Start early, plan carefully, write clearly and objectively Use outside (independent) readers to improve the quality of the proposal Read the RFA and instructions carefully, not just for deadlines Write the application with special attention to the Merit Criteria and goals of OOPD’s Natural History Grants program 5. Use OOPD’s Application Instructions and Helpful Hints 6. If you do not have expertise in certain areas e. g. , statistical analysis, pediatrics, etc, provide letters of collaboration for the needed expertise 7. Panel Reviewers are busy, so say it in fewer words if possible – DO NOT use appendices to circumvent the page limits 8. Ensure you work through grants. gov errors - it is the applicant’s responsibility to submit an error-free application by the deadline 9. Utilize the grants. gov review window to ensure the application appears as you intend it to be submitted 10. Call OOPD for program clarifications – 301 -796 -8660

Helpful Links OOPD Web Page: www. fda. gov/orphan Natural History Grant Program: http: //www. fda. gov/For. Industry/Developing. Productsfor. Rare. Diseases. Conditions/Orphan. Products. Natural. History. Grants. Program/default. htm RFA Link: http: //grants. nih. gov/grants/guide/rfa-files/RFA-FD-16 -043. html OOPD’s Application Instructions and Helpful Hints: http: //www. fda. gov/For. Industry/Developing. Productsfor. Rare. Diseases. Conditions/Orphan. Products. Natural. History. Grants. Program/ucm 512677. ht m Grants. gov: http: //grants. nih. gov/grants/submitapplication. htm http: //grants. nih. gov/grants/forms. htm http: //www. grants. gov/web/grants/applicant-faqs. html http: //grants. nih. gov/grants/policy/salcap_summary. htm http: //grants. nih. gov/grants/Electronic. Receipt/preparing. htm

Contacts Questions? We are here to help! Katherine Needleman, MS, Ph. D, RAC Director, Orphan Products Grants Programs Email: katherine. needleman@fda. hhs. gov Phone: (301) 796 -8664 Or Gumei Liu, MD, Ph. D Email: gumei. liu@fda. hhs. gov Phone: (301) 796 -0495
 Museum timelinefy
Museum timelinefy Noaa ago
Noaa ago Orphan boy and the elk dog
Orphan boy and the elk dog Intro to typography
Intro to typography Orphan annie eye nuclei
Orphan annie eye nuclei National orphan train museum
National orphan train museum Orphan movie
Orphan movie Stop that sulking at once amanda
Stop that sulking at once amanda Open orphan investor relations
Open orphan investor relations Orphan gpcr
Orphan gpcr Orphan annie hücreleri
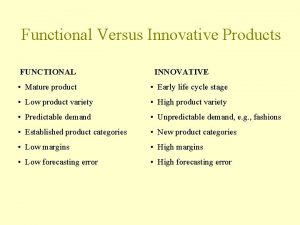
Orphan annie hücreleri Functional and innovative products examples
Functional and innovative products examples 4ps of pepsi
4ps of pepsi Mercantilism simplified
Mercantilism simplified Event based tourism product
Event based tourism product Npn site license
Npn site license Khawla natural products
Khawla natural products A business that gathers raw goods
A business that gathers raw goods Office software products
Office software products Natural hazards vs natural disasters
Natural hazards vs natural disasters Natural capital and natural income
Natural capital and natural income Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Frameset trong html5
Frameset trong html5 Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Tư thế worm breton là gì
Tư thế worm breton là gì Chúa yêu trần thế alleluia
Chúa yêu trần thế alleluia Môn thể thao bắt đầu bằng từ đua
Môn thể thao bắt đầu bằng từ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất