Oral Prostacyclin Pathway Agents in PAH 2018 in



![Therapies Targeting the Prostacyclin Pathway Therapy Class Route of Administration Epoprostenol[a] Prostanoid (synthetic prostacyclin) Therapies Targeting the Prostacyclin Pathway Therapy Class Route of Administration Epoprostenol[a] Prostanoid (synthetic prostacyclin)](https://slidetodoc.com/presentation_image_h2/3dd79bc0087b0c1293162b4cec4d38a5/image-6.jpg)

![FREEDOM Trials Oral Treprostinil Trial FREEDOM-M[a] FREEDOM-C[b] FREEDOM-C 2[c] FREEDOM-EV[d] N Background Therapy at FREEDOM Trials Oral Treprostinil Trial FREEDOM-M[a] FREEDOM-C[b] FREEDOM-C 2[c] FREEDOM-EV[d] N Background Therapy at](https://slidetodoc.com/presentation_image_h2/3dd79bc0087b0c1293162b4cec4d38a5/image-20.jpg)




- Slides: 24

Oral Prostacyclin Pathway Agents in PAH 2018 in Review Moderator Ioana R. Preston, MD Associate Professor of Medicine Director, Pulmonary Hypertension Center Tufts University School of Medicine Boston, Massachusetts, United States

Panelists David Langleben, MD Professor of Medicine, Division of Cardiology, Mc. Gill University Director, Center for Pulmonary Vascular Disease Jewish General Hospital Montreal, Quebec, Canada Nick H. Kim, MD Clinical Professor of Medicine Director of Pulmonary Vascular Medicine University of California San Diego La Jolla, California, United States

Background • Contemporary treatment recommendations for a patient with PAH is guided by – Evaluation – Risk stratification recommended by the 2015 ERS/ESC guidelines[a] • PAH is a progressive disease, which is (as of yet) incurable • While treating PAH in any patient is challenging, certain people tend to experience a more rapid progression; in such cases, PAH can be particularly difficult to manage a. Galiè N, et al. Eur Respir J. 2015; 46: 903 -975.

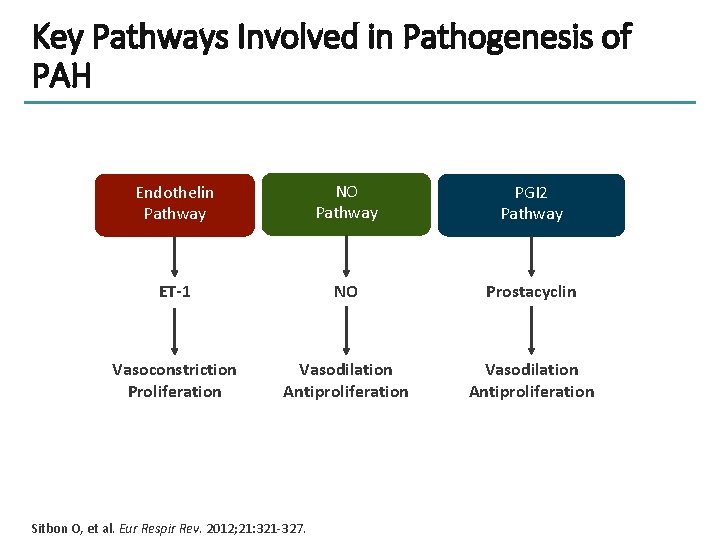
Key Pathways Involved in Pathogenesis of PAH Endothelin Pathway NO Pathway PGI 2 Pathway ET-1 NO Prostacyclin Vasoconstriction Proliferation Vasodilation Antiproliferation Sitbon O, et al. Eur Respir Rev. 2012; 21: 321 -327.

Pharmacology of Pathways Involved in Pathogenesis of PAH This material has not been reviewed by European Respiratory Society prior to release; therefore the European Respiratory Society may not be responsible for any errors, omissions or inaccuracies, or for any consequences arising there from, in the content. Reproduced with permission of the © ERS 2018. European Respiratory Review 24 (138): 630 -641; DOI: 10. 1183/16000617. 0067 -2015. Published 30 November 2015.
![Therapies Targeting the Prostacyclin Pathway Therapy Class Route of Administration Epoprostenola Prostanoid synthetic prostacyclin Therapies Targeting the Prostacyclin Pathway Therapy Class Route of Administration Epoprostenol[a] Prostanoid (synthetic prostacyclin)](https://slidetodoc.com/presentation_image_h2/3dd79bc0087b0c1293162b4cec4d38a5/image-6.jpg)
Therapies Targeting the Prostacyclin Pathway Therapy Class Route of Administration Epoprostenol[a] Prostanoid (synthetic prostacyclin) IV Iloprost[a] Prostanoid (prostacyclin analogue) IV Inhaled Treprostinil[a] Prostanoid (prostacyclin analogue) SC IV Inhaled Oral Beraprost[b] Prostanoid (Prostacyclin analogue) Oral Selexipag[c] Selective IP receptor agonist Oral a. Le. Varge BL. Ther Clin Risk Manag. 2015; 11: 535 -547; b. Galie N, et al. J Am Coll Cardiol. 2002; 39: 1496 -1502; c. Sitbon O, et al. N Engl J Med. 2015; 373: 2522 -2533.

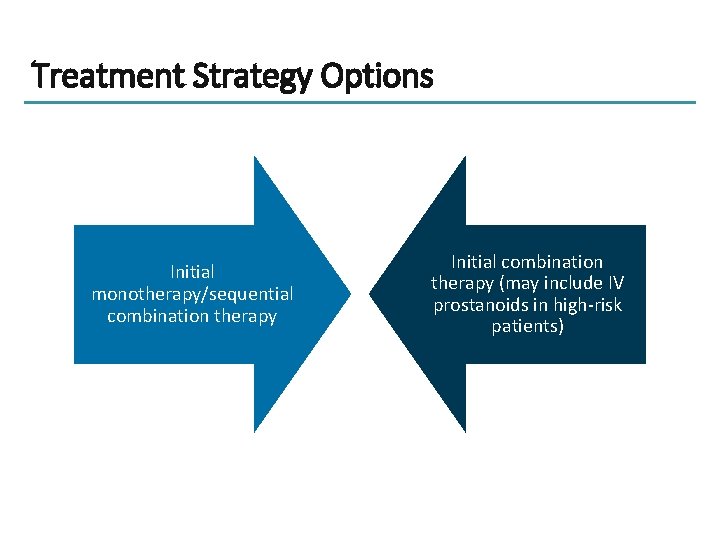
Treatment Strategy Options Initial monotherapy/sequential combination therapy Initial combination therapy (may include IV prostanoids in high-risk patients)

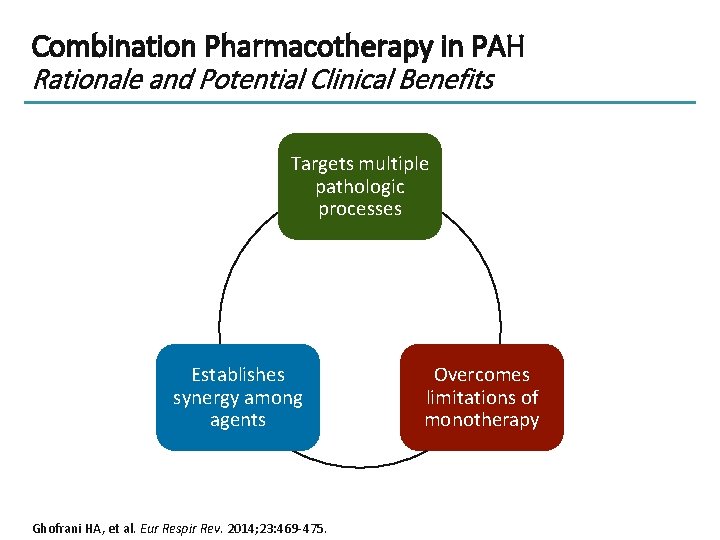
Combination Pharmacotherapy in PAH Rationale and Potential Clinical Benefits Targets multiple pathologic processes Establishes synergy among agents Ghofrani HA, et al. Eur Respir Rev. 2014; 23: 469 -475. Overcomes limitations of monotherapy

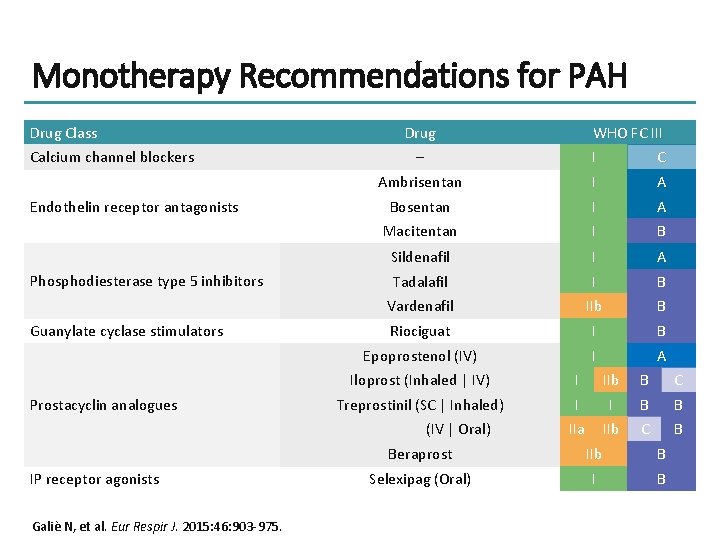
Monotherapy Recommendations for PAH Drug Class Calcium channel blockers Endothelin receptor antagonists Phosphodiesterase type 5 inhibitors Guanylate cyclase stimulators Prostacyclin analogues Drug – I C Ambrisentan I A Bosentan I A Macitentan I B Sildenafil I A Tadalafil I B Vardenafil IIb B Riociguat I B Epoprostenol (IV) I A Iloprost (Inhaled | IV) I IIb B C Treprostinil (SC | Inhaled) I I B B IIa IIb C B (IV | Oral) IP receptor agonists Galiè N, et al. Eur Respir J. 2015: 46: 903 -975. WHO FC III Beraprost IIb B Selexipag (Oral) I B

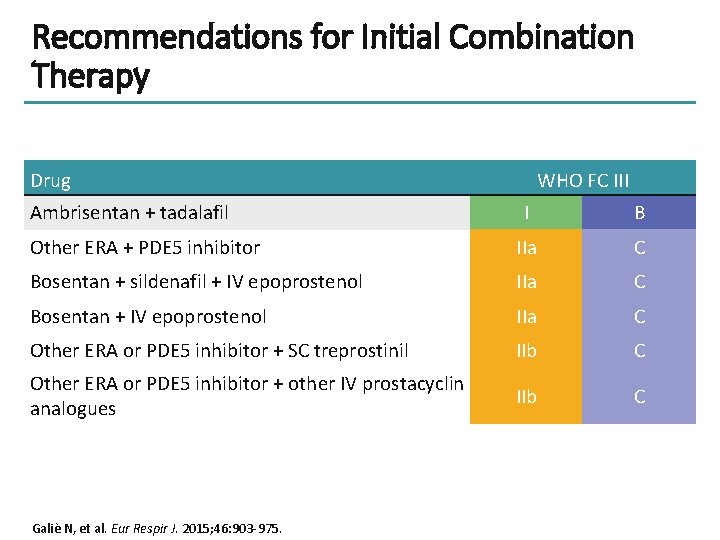
Recommendations for Initial Combination Therapy Drug Ambrisentan + tadalafil WHO FC III I B Other ERA + PDE 5 inhibitor IIa C Bosentan + sildenafil + IV epoprostenol IIa C Bosentan + IV epoprostenol IIa C Other ERA or PDE 5 inhibitor + SC treprostinil IIb C Other ERA or PDE 5 inhibitor + other IV prostacyclin analogues IIb C Galiè N, et al. Eur Respir J. 2015; 46: 903 -975.

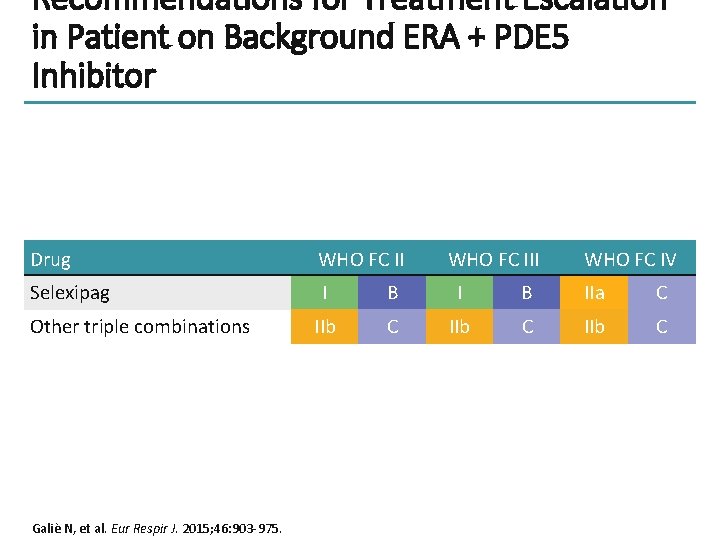
Recommendations for Treatment Escalation in Patient on Background ERA + PDE 5 Inhibitor Drug WHO FC II Selexipag I B IIa C IIb C Other triple combinations Galiè N, et al. Eur Respir J. 2015; 46: 903 -975. WHO FC III WHO FC IV

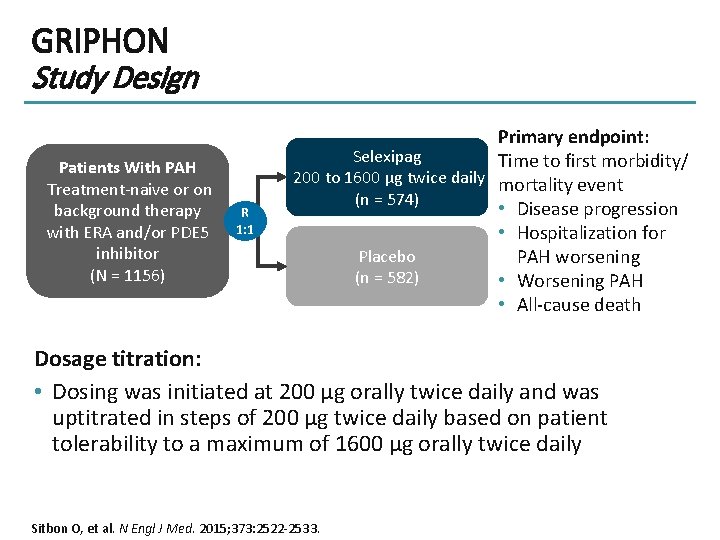
GRIPHON Study Design Patients With PAH Treatment-naive or on background therapy with ERA and/or PDE 5 inhibitor (N = 1156) R 1: 1 Primary endpoint: Selexipag Time to first morbidity/ 200 to 1600 µg twice daily mortality event (n = 574) • Disease progression • Hospitalization for Placebo PAH worsening (n = 582) • Worsening PAH • All-cause death Dosage titration: • Dosing was initiated at 200 μg orally twice daily and was uptitrated in steps of 200 μg twice daily based on patient tolerability to a maximum of 1600 μg orally twice daily Sitbon O, et al. N Engl J Med. 2015; 373: 2522 -2533.

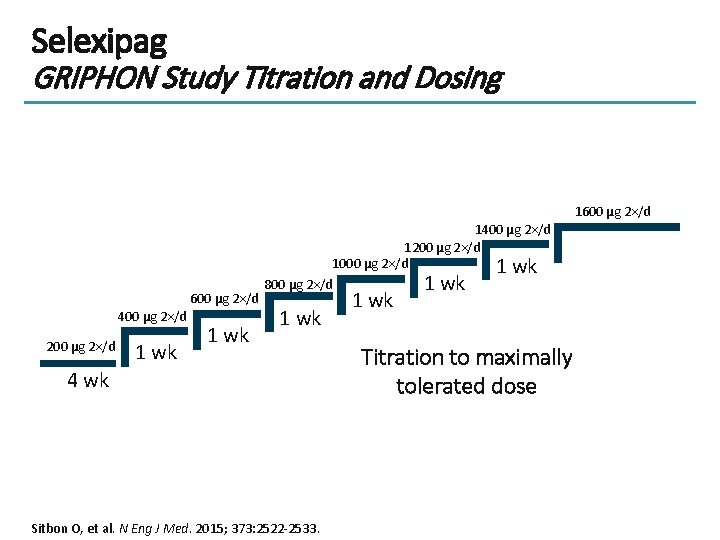
Selexipag GRIPHON Study Titration and Dosing 1600 μg 2×/d 400 μg 2×/d 200 μg 2×/d 1 wk 1400 μg 2×/d 1200 μg 2×/d 1000 μg 2×/d 1 wk 800 μg 2×/d 1 wk 4 wk Sitbon O, et al. N Eng J Med. 2015; 373: 2522 -2533. 1 wk Titration to maximally tolerated dose

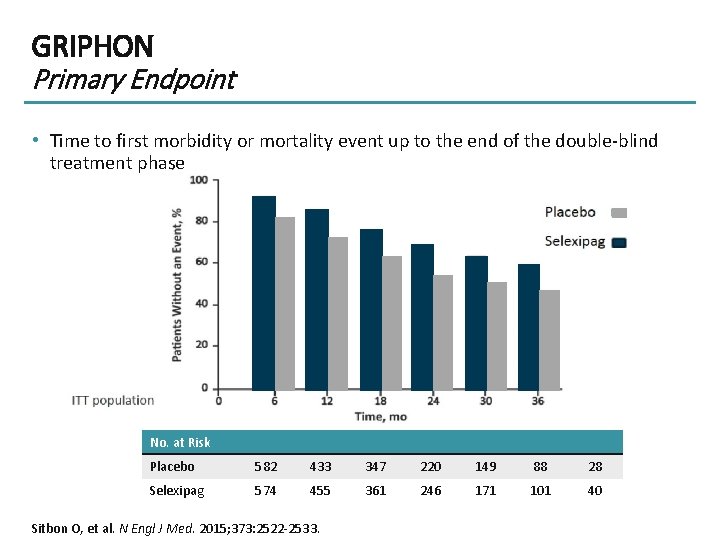
GRIPHON Primary Endpoint • Time to first morbidity or mortality event up to the end of the double-blind treatment phase No. at Risk Placebo 582 433 347 220 149 88 28 Selexipag 574 455 361 246 171 101 40 Sitbon O, et al. N Engl J Med. 2015; 373: 2522 -2533.

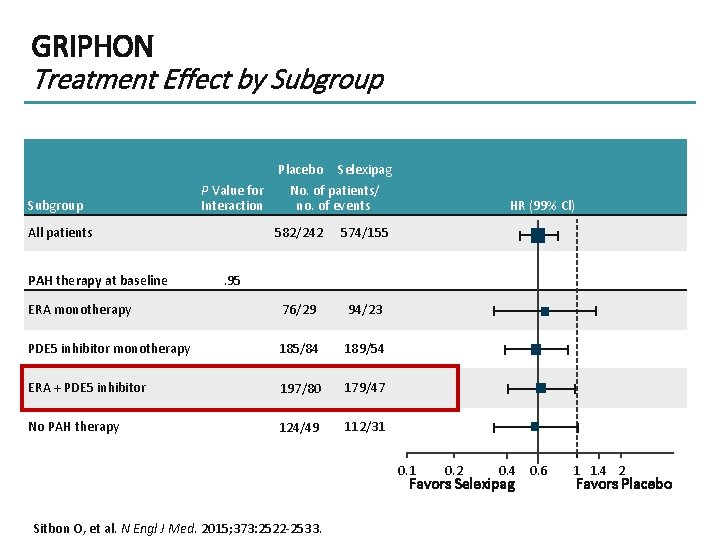
GRIPHON Treatment Effect by Subgroup P Value for Interaction All patients Placebo Selexipag No. of patients/ no. of events 582/242 574/155 ERA monotherapy 76/29 94/23 PDE 5 inhibitor monotherapy 185/84 189/54 ERA + PDE 5 inhibitor 197/80 179/47 No PAH therapy 124/49 112/31 PAH therapy at baseline HR (99% Cl) . 95 0. 1 0. 2 0. 4 0. 6 Favors Selexipag Sitbon O, et al. N Engl J Med. 2015; 373: 2522 -2533. 1 1. 4 2 Favors Placebo

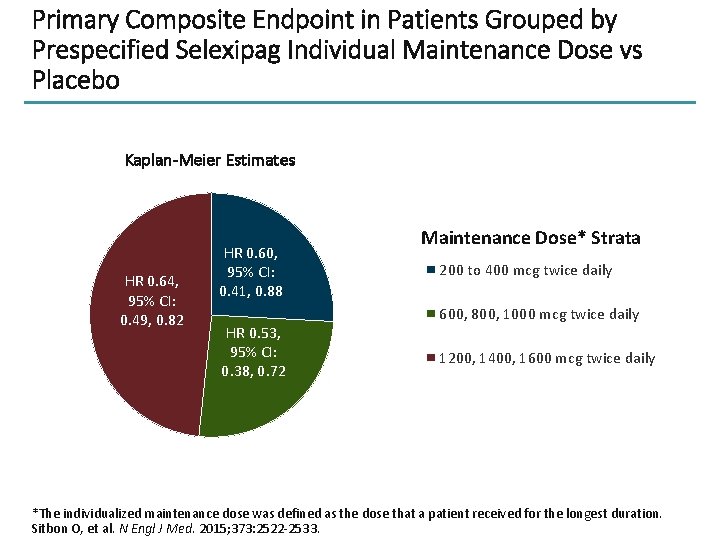
Primary Composite Endpoint in Patients Grouped by Prespecified Selexipag Individual Maintenance Dose vs Placebo Kaplan-Meier Estimates HR 0. 64, 95% CI: 0. 49, 0. 82 HR 0. 60, 95% CI: 0. 41, 0. 88 HR 0. 53, 95% CI: 0. 38, 0. 72 Maintenance Dose* Strata 200 to 400 mcg twice daily 600, 800, 1000 mcg twice daily 1200, 1400, 1600 mcg twice daily *The individualized maintenance dose was defined as the dose that a patient received for the longest duration. Sitbon O, et al. N Engl J Med. 2015; 373: 2522 -2533.

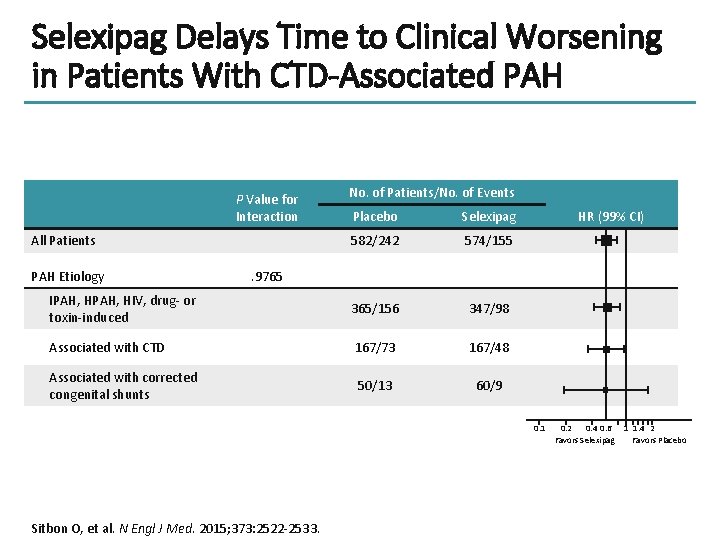
Selexipag Delays Time to Clinical Worsening in Patients With CTD-Associated PAH P Value for Interaction No. of Patients/No. of Events Placebo Selexipag 582/242 574/155 IPAH, HIV, drug- or toxin-induced 365/156 347/98 Associated with CTD 167/73 167/48 Associated with corrected congenital shunts 50/13 60/9 All Patients PAH Etiology HR (99% CI) . 9765 0. 1 Sitbon O, et al. N Engl J Med. 2015; 373: 2522 -2533. 0. 2 0. 4 0. 6 Favors Selexipag 1 1. 4 2 Favors Placebo

GRIPHON Connective Tissue Disease-Associated PAH Figure No Longer Available This material has not been reviewed by European Respiratory Society prior to release; therefore the European Respiratory Society may not be responsible for any errors, omissions or inaccuracies, or for any consequences arising there from, in the content. Reproduced with permission of the © ERS 2018. European Respiratory Review 50(2): pii: 1602493. DOI: 10. 1183/13993003. 02493 -2016. Published 17 August 2017. Modified N Eng Med J, humbert, et al. , Treatment of Pulmonary Arterial Hypertension, 351, 1425 -1436. Copyright © 2004 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.

GRIPHON Temporary Treatment Interruptions Treatment interruptions were allowed • if the interruption was < 3 days, treatment was restarted at the previous highest tolerated dose • if the interruption was ≥ 3 days, retitration from 200 µg twice daily was required RESULTS: • ≥ 1 treatment interruption occurred in 19. 3% in the selexipag group vs 10% in placebo group – 85% of patients in selexipag group were receiving background PAH therapy • Baseline characteristics were similar between patients with and without an interruption • Adverse events were the most common reason for selexipag interruption • Selexipag interruptions and reinstitution of treatment were well tolerated • There were no episodes of acute deterioration during treatment interruption Preston IR, et al. J Heart Lung Transplant. 2018; 37: 401 -408.
![FREEDOM Trials Oral Treprostinil Trial FREEDOMMa FREEDOMCb FREEDOMC 2c FREEDOMEVd N Background Therapy at FREEDOM Trials Oral Treprostinil Trial FREEDOM-M[a] FREEDOM-C[b] FREEDOM-C 2[c] FREEDOM-EV[d] N Background Therapy at](https://slidetodoc.com/presentation_image_h2/3dd79bc0087b0c1293162b4cec4d38a5/image-20.jpg)
FREEDOM Trials Oral Treprostinil Trial FREEDOM-M[a] FREEDOM-C[b] FREEDOM-C 2[c] FREEDOM-EV[d] N Background Therapy at Baseline Treprostinil Dosage Primary Endpoint Significant improvement in 6 MWD (P =. 0125)* 349 None Dose-titrated (mean: 3. 4 mg) twice daily, 12 weeks 350 ERA and/or PDE 5 inhibitor Dose-titrated (median: 3. 0 mg) twice daily, 16 weeks No significant improvement in 6 MWD (P =. 07)* 310 ERA and/or PDE 5 inhibitor Dose-titrated (mean: 3. 1 mg) twice daily, 16 weeks No significant improvement in 6 MWD (P =. 89)* ERA/PDE 5 inhibitor/s. GC Dose-titrated (initiated at 0. 125 mg to a maximum of 12 mg) three times daily, 24 weeks 690 26% reduction in risk of morbidity/mortality (P =. 039) *Placebo-corrected change in 6 MWD from baseline to end of study period. a. Jing ZC, et al. Circulation. 2013; 127: 624 -633; b. Tapson VF, et al. Chest. 2012; 142: 1383 -1390; c. Tapson VF, et al. Chest. 2013; 144: 952 -958; d. United Therapeutics (2018) [Press release].

Unresolved Issues With Using Oral vs Parenteral Routes • FC IV patients with low CO: Infusion therapies recommended • Unsure about dose equivalence • Unsure about potency equivalence • Unclear whether transition can be successfully accomplished • Lack of safety and efficacy data

Patient Case • • • • 45 -year-old woman, recently arrived in Canada from Pakistan Hypertensive, diabetic, dyslipidemia Presented November 2017 with 1 year dyspnea, worsening to late FC III in last month No other risk factor Exam consistent with severe PH Echocardiogram -- dilated, severely hypokinetic RV PFTs, VQ, and CT of chest show no contributing abnormality Catheterization: LVEDP 14 mm. Hg, RAP 10 mm. Hg, PAP 121/40 mm. Hg, mean 67, PAWP 10 mm. Hg, Cardiac output 5. 1 L/min, cardiac index 2. 7 L/min/m 2 , circumferential pericardial effusion NT-pro. BNP 562 ng/L, 6 MWD 260 m Presumed diagnosis: IPAH Started on tadalafil and macitentan November 2017 Feb 2018: a bit better but still FC III, NT-pro. BNP 425 ng/L Started on selexipag, uptitrated to 1400 mcg bid Month 3 of selexipag: NT-pro. BNP 82 ng/L, FC II, 6 MWD 370 m – Echocardiogram – improved RV size and function, no pericardial effusion • Month 6 of selexipag NT-pro. BNP 92 ng/L, FC II, 6 MWD 366 m

Multidisciplinary Approach to PAH Management Specialists Cardiologists Pulmonologists Rheumatologists Radiologists Transplantation surgeons Patient Support Groups PAH Nurses Other HCPs Primary Care Providers Galiè N, et al. Eur Respir J. 2016; 37: 67 -119. Occupational therapists Physical therapists Dieticians Pharmacists Psychologists

Thank you for participating in this Thank you foractivity. participating in this activity. Please proceed to answer the post-activity assessment questions and receive credit. Please also take a moment to complete the program evaluation.
 Prostacyclin
Prostacyclin Ww2 phonetic alphabet
Ww2 phonetic alphabet Med
Med Pah in echo
Pah in echo Pah clearance
Pah clearance Pah groups
Pah groups Pah clearance
Pah clearance Que letra continua m v t m j
Que letra continua m v t m j Douglas wilhelm harder
Douglas wilhelm harder Inotropic agents
Inotropic agents Language artinya
Language artinya Types of travel agents
Types of travel agents Antimycobacterial agents
Antimycobacterial agents Differentiate between oxidizing and reducing agents
Differentiate between oxidizing and reducing agents Hydrolysis feldspar
Hydrolysis feldspar Calcipotiene
Calcipotiene Oxidizing agent chart
Oxidizing agent chart Types of agents in artificial intelligence
Types of agents in artificial intelligence Knowledge-based agents
Knowledge-based agents Goanywhere agent
Goanywhere agent Agents of socialization
Agents of socialization Antimicrotubule agents
Antimicrotubule agents Examples of mechanical weathering
Examples of mechanical weathering 5 agents in developing personality
5 agents in developing personality Formal agents of social control
Formal agents of social control