Optimizing Organ Donation Donor Alliance Organ Donation Summit





















- Slides: 48


Optimizing Organ Donation Donor Alliance Organ Donation Summit December 8, 2015 Mary Laird Warner, MD, FCCP Chairman, Quality Medical Executive Committee Swedish Medical Center Associate Professor, Critical Care and Pulmonary Medicine, National Jewish Health

Disclosures - I have no financial conflicts of interest to disclose. Level of evidence - Levels 1, 2, 3

Outline • Discuss the scope of organ donation needs • Review physiologic changes of brain death • Discuss ICU care of the brain dead organ donor • Discuss bundling of Donor Management Goals (DMG) • Review results from DMG protocol at Swedish Medical Center

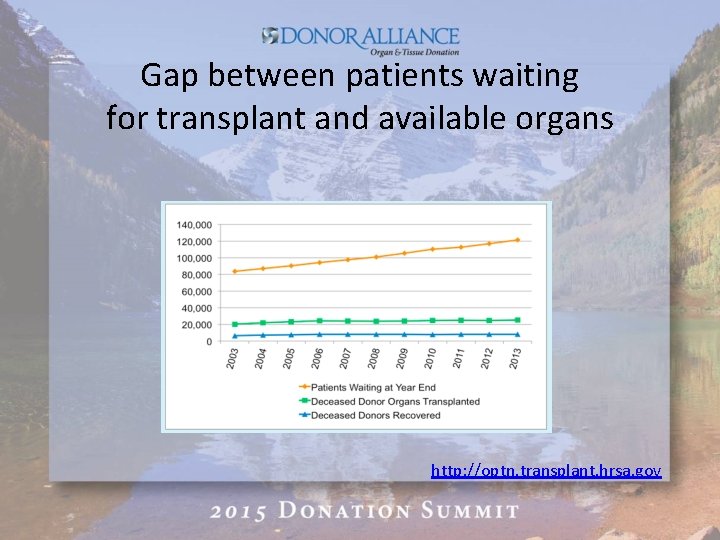
The Need • Every 10 minutes, someone is added to the national organ transplant waiting list. • On average, 22 people die each day waiting for an organ transplant. • The gap between supply and demand of transplanted organs continues to widen. http: //optn. transplant. hrsa. gov Updated 11. 27. 2015

Gap between patients waiting for transplant and available organs http: //optn. transplant. hrsa. gov

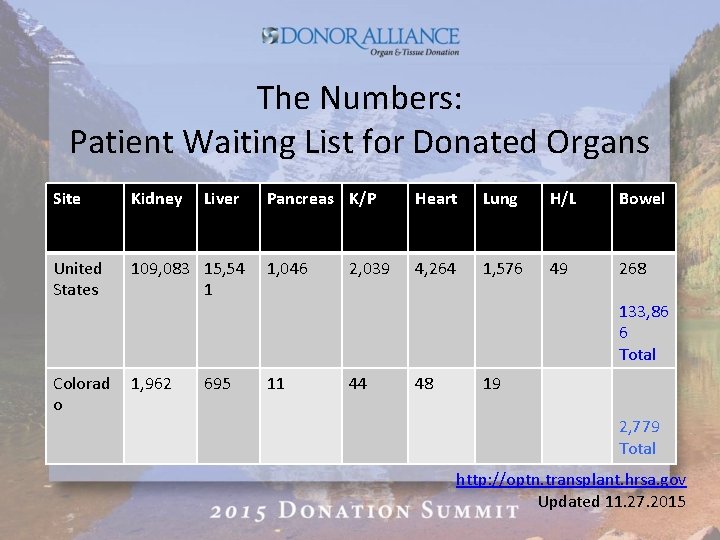
The Numbers: Patient Waiting List for Donated Organs Site Kidney United States Colorad o Liver Pancreas K/P Heart Lung H/L Bowel 109, 083 15, 54 1 1, 046 4, 264 1, 576 49 268 1, 962 11 695 2, 039 133, 86 6 Total 44 48 19 2, 779 Total http: //optn. transplant. hrsa. gov Updated 11. 27. 2015

How can we bridge the donor gap? Maximize the number of organs transplanted per donor (OTPD) Goal per HHS/ HRSA = 3. 75

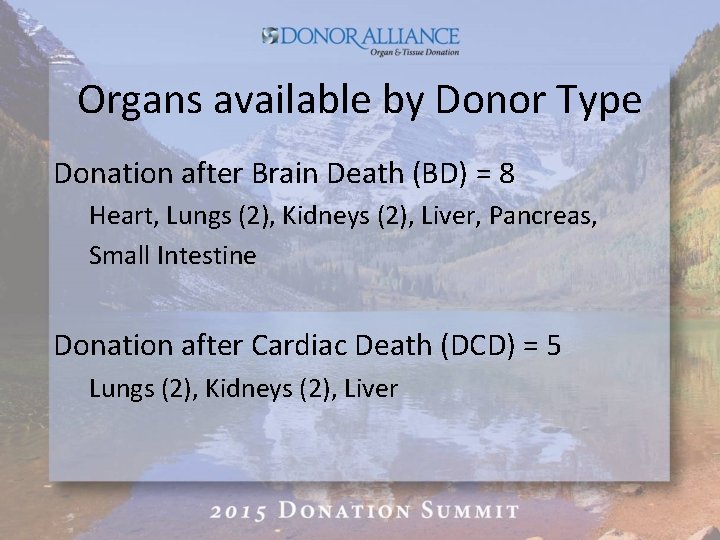
Organs available by Donor Type Donation after Brain Death (BD) = 8 Heart, Lungs (2), Kidneys (2), Liver, Pancreas, Small Intestine Donation after Cardiac Death (DCD) = 5 Lungs (2), Kidneys (2), Liver

Physiologic Changes with Brain Death Cardiovascular Metabolic Neurologic Endocrine Pulmonary

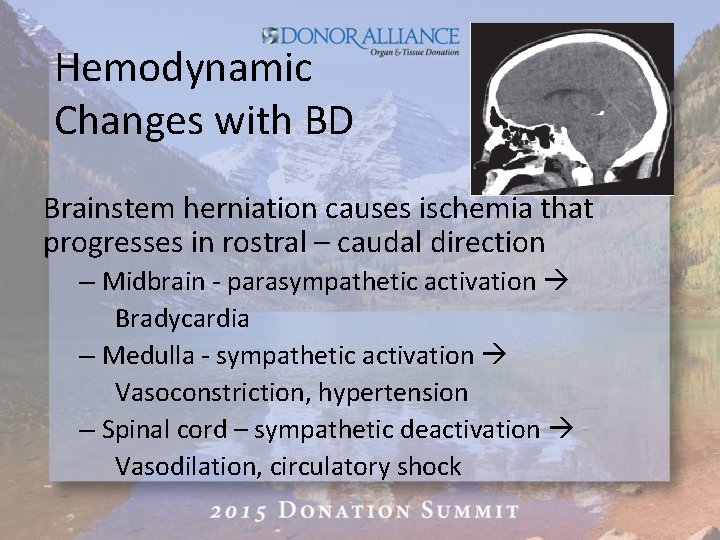
Hemodynamic Changes with BD Brainstem herniation causes ischemia that progresses in rostral – caudal direction – Midbrain - parasympathetic activation Bradycardia – Medulla - sympathetic activation Vasoconstriction, hypertension – Spinal cord – sympathetic deactivation Vasodilation, circulatory shock

Hemodynamic changes with BD • 10 -20% donors are lost to cardiovascular collapse as patient evolves to brain death • 50% of potential BD donors are volume responsive • Pro-inflammatory state, increased cytokine IL-6 • Resultant shock causes – Progressive organ failure – Fewer OTPD – Lower survival of transplanted organs Muragan, CCM, 2009

Volume Depletion in BD • Causes multifactorial – Underlying medical condition – blood loss, etc – Prior management – osmotic therapy for ICP – Neuro-hormonal cascade – Capillary Leak – Diabetes Insipidus

Pulmonary Changes in BD • Pulmonary edema – Neurogenic – Cardiogenic – Non-cardiogenic – capillary endothelial leak – Delayed alveolar fluid clearance

Hormonal changes with BD • Catecholamine storm • Ischemia to pituitary and hypothalamus depletes AVP, cortisol, thyroid hormones – Diabetes insipidus – up to 90% BD patients – Hypocortisolism – Hyothyroidism

Metabolic changes with BD • Hypernatremia – Caused by volume depletion, Diabetes insipidus – Na > 170 associated primary non-function (PNF) of graft liver • Hyperglycemia – Caused by insulin resistance and gluconeogenesis – Glu > 200 associated with PNF of graft pancreas – Glu > 160 associated with PNF of graft kidney

ICU Management of the Brain Dead Potential Donor • Stabilize profound physiologic and homeostatic derangements provoked by BD • Balance competing management priorities between different organs • Avert somatic death and loss of all organs

Management of the potential organ donor in the ICU: SCCM, ACCP, AOPO Consensus Statement Critical Care Medicine 2015 • First US expert report • Multidisciplinary, multi-institutional • Review of available evidence from observational studies and case series • Form of consensus statement • Practical guideline for care of organ donor Crit Care Med 2015; 43: 1291 -1325

Circulatory support • Physiologic goals – Target euvolemia – MAP > 60 mm Hg – UOP > 1 m. L/kg/hr – EF > 45% – Low pressor dose – Dopamine 1 -10 mcg/kg/hr • Volume Resuscitation – Crystalloid – NS or LR - for volume replacement – Colloids – for acute volume expansion – Avoid Hydroxyethyl starch (HES)

Vasoactive Medications - Pressors Dopamine • Traditional 1 st line pressor • 1 -10 mcg/kg/min • Inotrope and vasopressor • Pro – suppresses inflammation; mitigates ischemia-reperfusion injury • Con – suppresses anterior pituitary hormone function Vasopressin • Alternative 1 st line pressor • . 01 -. 04 IU/min • Vasoconstrictor • Pro – catecholamine sparing effect; concurrent rx of DI • Con - Decreases splanchnic perfusion

Vasoactive Medications - Inotropes • If EF < 45% despite volume repletion and pressors, add inotrope – Dobutamine, Epinephrine • If EF remains depressed despite inotrope, consider starting hormonal replacement therapy (HRT)

Hemodynamic Monitoring • Static measurement – Central venous, PA catheter – Sc. VO 2 – Lactate – Base deficit – Serial CVP, PAOP, CO, CI • Dynamic measurements – Pulse contour analysis • Echo – Transthoracic (TTE) vs Transesophagael (TEE) – EF

Vasoactive support: Treating AVP deficiency • Hypotension despite volume repletion – AVP - Vasopressin -. 01 -. 04 IU/min • Diabetes insipidus (DI) – – UOP > 3 m. L/kg/hr U Osm < 200 m. Osm/kg H 2 O or S Osm > 305 Serum Na > 145 mmol/L Desmopressin – 1 -4 mcg IVP, then 2 mcg IV Q 6 hrs • AVP treatment associated with – Decreased pressor and inotrope need – increased rate of organ recovery

Corticosteroids for vasoactive support CORTISOME study: Prospective, randomized study of low dose hydrocortisone effect on resuscitation of BD donors Subjects: 259 BD organ donors Intervention: Hydrocortisone (HC) vs none Results: Patients receiving HC had Lower dose and shorter duration pressor needs No difference in transplantation or graft survival Pinsard, 2014

Corticosteroid Therapy for Immunomodulation • Corticosteroid repletion reduces inflammation in donor livers – – Lower levels pro-inflammatory cytokines – serum, tissue Fewer adhesion molecules in tissue Kotsch, 2008 Less ischemia-reperfusion injury Lower acute rejection rates • Conflicting results in cardiac, lung, renal grafts • Recommended dose: Methylprednisolone – 1000 mg IV, 250 mg IV, or 15 mg/kg IV bolus – 100 mg/hr IV infusion

Thyroid hormone replacement for vasoactive support Conflicting evidence over years: 16 retrospective studies / case series suggested T 3/T 4 infusion improved cardiac index Meta- analysis of 4 RCTs of 209 donors No effect on cardiac index Recommendation: Consider thyroid replacement for hemodynamically unstable BD donors or for EF < 45% Dose: T 4: 20 mcg IV bolus, 10 mcg/hr IV gtt T 3: 4 mcg IV bolus, 3 mcg/hr IV gtt Mac. Donald, 2012

3 hormone replacement for vasoactive support of BD Donor Multicenter, retrospective study by UNOS of 10, 292 BD donors using 3 -drug HRT cocktail (AVP, methylprednisolone, T 3/T 4) • 22. 5% higher OTPD compared to controls • Increased probability organ donation – Kidney, heart, liver, lung and pancreas • Increased probability of organ survival at 1 year Mason, 1993

Insulin therapy in BD • Glucose > 160 associated with reduced posttransplant renal graft function • Glucose > 200 associated with reduced posttransplant pancreas graft function • Potential to deplete graft B-islet cells • No large studies to guide recommendations • Recommend: Serum glucose < 180 mg/d. L

Pulmonary support • Physiologic goals – Arterial p. H 7. 3 – 7. 45 – Pa. O 2/Fi. O 2 > 300 • Avoid excessive fluid resuscitation – Target CVP 4 – 6 mm Hg • Avoid Vasopressors • Ventilator strategy – Conventional – high VT 10 -12 m. L/kg IBW + low PEEP – Lung Protective – low VT 6 m. L/kg IBW + mod PEEP

Lung Protective Ventilation in BD organ donors RCT of 118 BD patients randomized to 6 hrs of randomized vs conventional ventilation Lung protective ventilation: (VT 6 -8 m. L/kg IBW + PEEP 8 -10 cm H 2 O) + recruitment maneuvers + apnea test on CPAP Conventional ventilation: (VT 10 -12 m. L/kg + PEEP 5 cm H 2 O) + no recruitment maneuvers + apnea test off ventilator Results: Lung protective ventilation resulted in Higher percentage transplantable lungs (95 vs 54%, P < 0. 001) Higher number lungs transplanted (54 vs 27%, p = 0. 004) Lower inflammatory biomarkers (IL-6, Soluble TNF receptors) Mascia, 2010

Salvage maneuvers to improve lung recovery for transplantation Donor management protocol improve lung recovery rate 3 fold • Conservative fluid strategy/ diuresis • Lung recruitment maneuvers • Early therapeutic bronchoscopy • Chest physiotherapy Q 4 hrs Salvage ventilator modes improve lung recovery rate 3 -4 fold – Bilevel 25/15 – Airway pressure release ventilation (APRV) Gabbay, 1999 Angel, 2006 Hanna, 2001

Renal Support • Physiologic goals - Euvolemia – CVP 4 -10 mm Hg – UOP > 1 m. L/kg/hr • Resuscitate with crystalloids or colloids • Avoid HES – Delayed graft function – Elevated serum Cr at PTD 10 • Single, low-dose pressor use – Dopamine as pressor of choice – AVP may increase renal procurement

Effect of donor pretreatment with dopamine on graft function after kidney transplantation RCT, open-label, parallel study of 264 brain dead donors of 487 kidneys from 60 European centers, 2004 -2007. Intervention: low dose DA = 4 mcg/kg/min vs none Results: Donors receiving dopamine were less likely to require dialysis (24. 7% vs 35. 4%, p 0. 01) Multiple dialyses associated with renal graft failure at 3 years. HR 3. 61 (2. 39 -5. 45) Schnuelle, 2009

Organ-specific management: Liver • Na > 155 in graft liver risks swelling upon transplantation • Na > 155 in donor associated with – Increased need for re-transplantation at 30 d – Increased allograft failure at 90 d • Recommend: Serum Na < 155

Organ-specific management: Pancreas and Small Intestine • Target Euvolemia • Provide 3 x HRT – enhances pancreatic utilization • Target serum glucose < 180 mg/d. L • Continue enteral nutrition • Prophylactic antibiotics Small bowel decontamination regimen Broad-spectrum iv antibiotic prophylaxis • Avoid use of SB from patients with prolonged shock/ resuscitation or GI bleeding

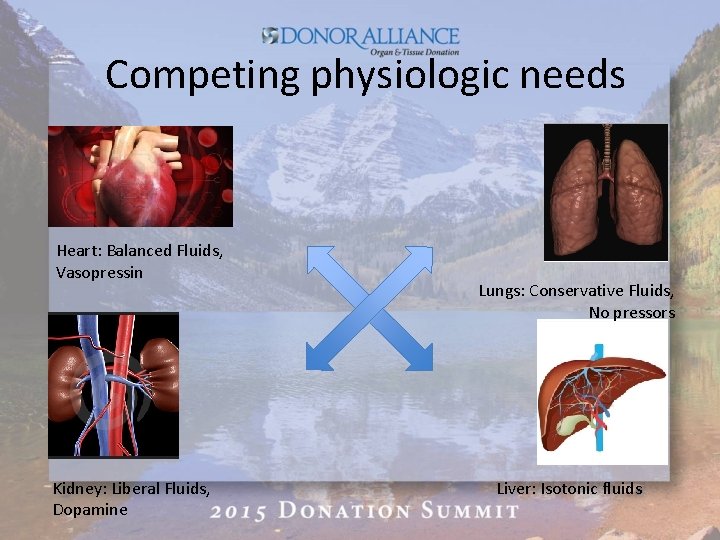
Competing physiologic needs Heart: Balanced Fluids, Vasopressin Kidney: Liberal Fluids, Dopamine Lungs: Conservative Fluids, No pressors Liver: Isotonic fluids

Protocols to maximize OPTD • Donor Management Goals (DMGs) • Order sets • Intensivist-led organ donor management

Donor Management Goals (DMG) • Develop protocols to optimize donor organ function and maximize OTPD • Borrow concept of “bundles” from other disease management models • Represent consensus of physiologic targets based on expert opinion • Modest clinical studies to support use

Donor management goals MAP 60 – 100 mm Hg ABG p. H 7. 30 – 7. 45 CVP 4 – 10 mm Hg Pa. O 2: Fi. O 2 > 300 EF > 50% Na 135 - 160 m. Eq/L Pressor < 1; low dose Glu < 150 mg/d. L Thyroid hormone UOP 0. 5 – 3 m. L/kg/hr J Trauma. 2011 Oct; 71(4): 990 -5 Crit Care Med. 2012 Oct; 40(10): 2773 -80 Am Surg 2010 Jun; 76 (6): 587 -94

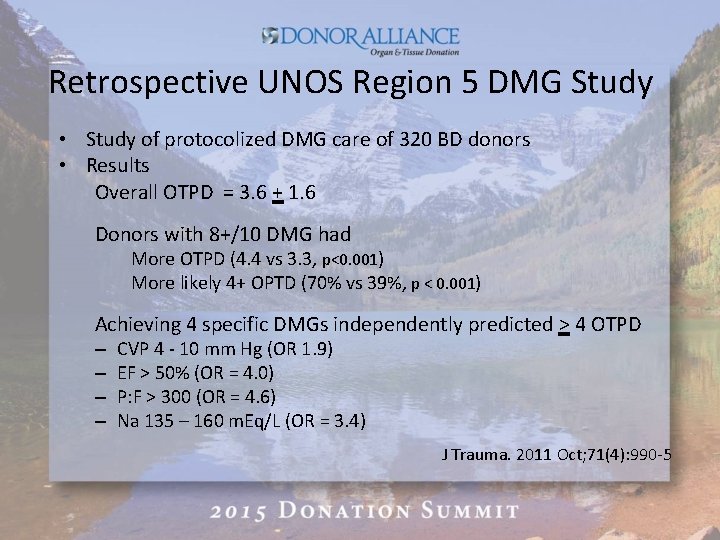
Retrospective UNOS Region 5 DMG Study • Study of protocolized DMG care of 320 BD donors • Results Overall OTPD = 3. 6 + 1. 6 Donors with 8+/10 DMG had More OTPD (4. 4 vs 3. 3, p<0. 001) More likely 4+ OPTD (70% vs 39%, p < 0. 001) Achieving 4 specific DMGs independently predicted > 4 OTPD – – CVP 4 - 10 mm Hg (OR 1. 9) EF > 50% (OR = 4. 0) P: F > 300 (OR = 4. 6) Na 135 – 160 m. Eq/L (OR = 3. 4) J Trauma. 2011 Oct; 71(4): 990 -5

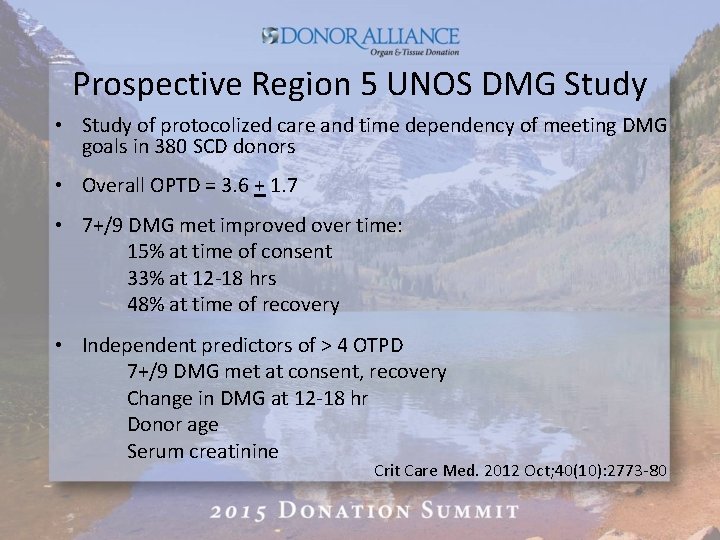
Prospective Region 5 UNOS DMG Study • Study of protocolized care and time dependency of meeting DMG goals in 380 SCD donors • Overall OPTD = 3. 6 + 1. 7 • 7+/9 DMG met improved over time: 15% at time of consent 33% at 12 -18 hrs 48% at time of recovery • Independent predictors of > 4 OTPD 7+/9 DMG met at consent, recovery Change in DMG at 12 -18 hr Donor age Serum creatinine Crit Care Med. 2012 Oct; 40(10): 2773 -80

UNOS Region 11 Prospective DMG Study • Prospective study of 805 SCD donors, 2685 total, including ECD, DCD • OPTD – 2. 85 - 2. 96 when < 8 DMGs met – 3. 34 - 3. 44 when all 8 DMGs met • Lung transplants increased 2. 4 x when all 8 DMGs met • DMGs associated with highest OTPD – Low VP use – P: F > 200 – CVP 4 -10 • DMGs associated with recovery of specific organs – Heart: Na, low VP use – Lung: CVP, P: F – Pancreas: glucose control Am Surg 2010 Jun; 76 (6): 587 -94

Intensivist-led management • • University of Pittsburgh study Intensivist-led organ donor support team Pre-post study design (n= 35 pre/ 45 post) Results – Increased total organs recovered (66/210 vs 113/258) – Increased lungs recovered (8/70 vs 21/86) – Increased kidneys recovered (31/70 vs 52/86) – Increased OTPD ( 1. 9 vs 2. 6) Singbartl, 2011

Future Directions • MOni. To. R Trial – Monitoring Organ donors to improve Transplant Results Alkhafaji, R, 2015 • Glycemic control – conventional vs intensive glycemic control effects on renal graft function Niemmann, in press

Organ Viability Research Project at Swedish Medical Center • Focus: Collect data of 9 DMGs from time of BD declaration to DA management • Goal: Develop protocol for donor management to increase OTPD > 3. 75 • Scope: – Baseline study period 2010 -2012 – Prospective study, starting June 2013

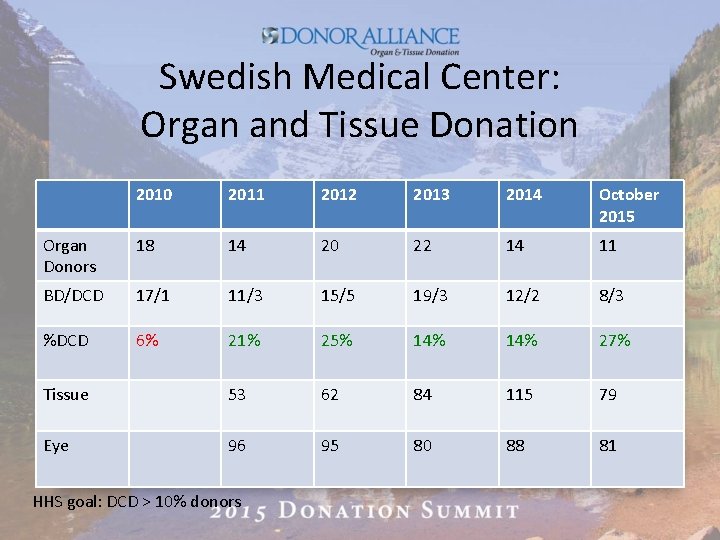
Swedish Medical Center: Organ and Tissue Donation 2010 2011 2012 2013 2014 October 2015 Organ Donors 18 14 20 22 14 11 BD/DCD 17/1 11/3 15/5 19/3 12/2 8/3 %DCD 6% 21% 25% 14% 27% Tissue 53 62 84 115 79 Eye 96 95 80 88 81 HHS goal: DCD > 10% donors

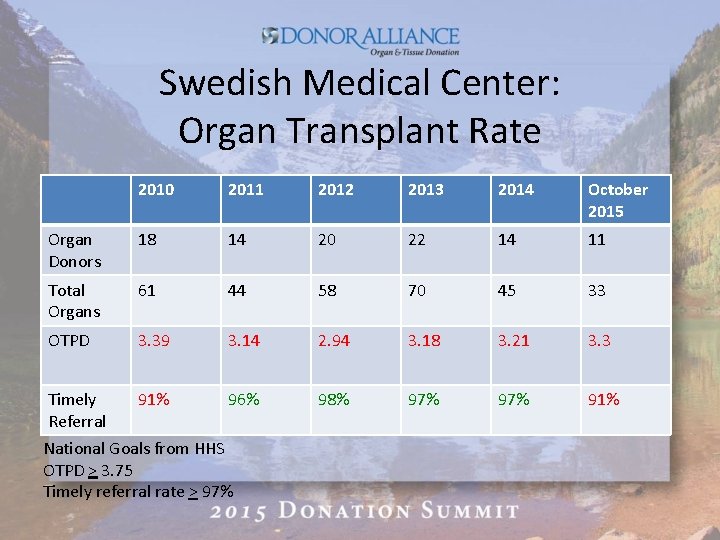
Swedish Medical Center: Organ Transplant Rate 2010 2011 2012 2013 2014 October 2015 Organ Donors 18 14 20 22 14 11 Total Organs 61 44 58 70 45 33 OTPD 3. 39 3. 14 2. 94 3. 18 3. 21 3. 3 Timely Referral 91% 96% 98% 97% 91% National Goals from HHS OTPD > 3. 75 Timely referral rate > 97%

Questions? Thank You