NSW Department of Education Stage 6 Chemistry Module










- Slides: 34

NSW Department of Education Stage 6 Chemistry Module 7 IQ 4 How can alcohols be produced and what are their properties?

NSW Department of Education How can alcohols be produced and what are their properties? • Inquiry question - How can alcohols be produced and what are their properties? • This document contains topic specific content notes to support students. In addition, several self-assessment opportunities are presented for this inquiry question with HSC-style items allowing students to review their responses with regards to the marking criteria. 2

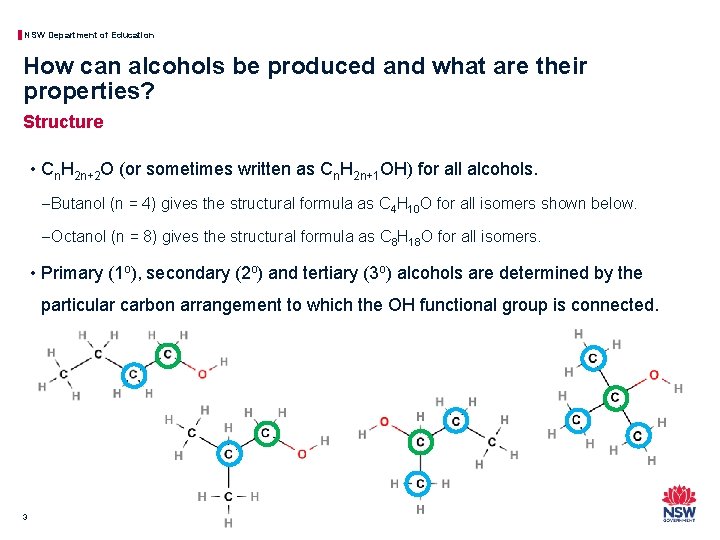
NSW Department of Education How can alcohols be produced and what are their properties? Structure • Cn. H 2 n+2 O (or sometimes written as Cn. H 2 n+1 OH) for all alcohols. − Butanol (n = 4) gives the structural formula as C 4 H 10 O for all isomers shown below. − Octanol (n = 8) gives the structural formula as C 8 H 18 O for all isomers. • Primary (1 o), secondary (2 o) and tertiary (3 o) alcohols are determined by the particular carbon arrangement to which the OH functional group is connected. 3

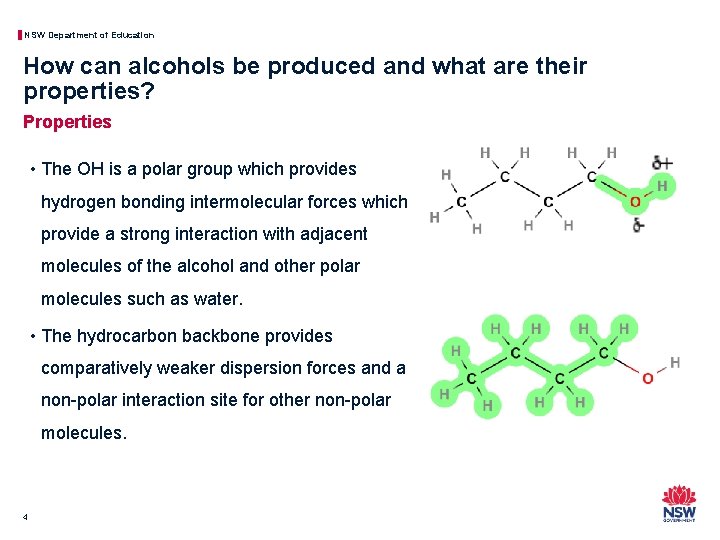
NSW Department of Education How can alcohols be produced and what are their properties? Properties • The OH is a polar group which provides hydrogen bonding intermolecular forces which provide a strong interaction with adjacent molecules of the alcohol and other polar molecules such as water. • The hydrocarbon backbone provides comparatively weaker dispersion forces and a non-polar interaction site for other non-polar molecules. 4

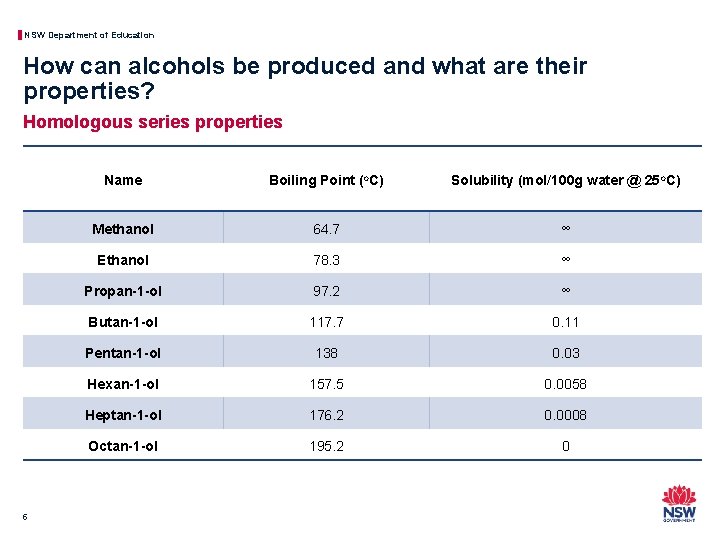
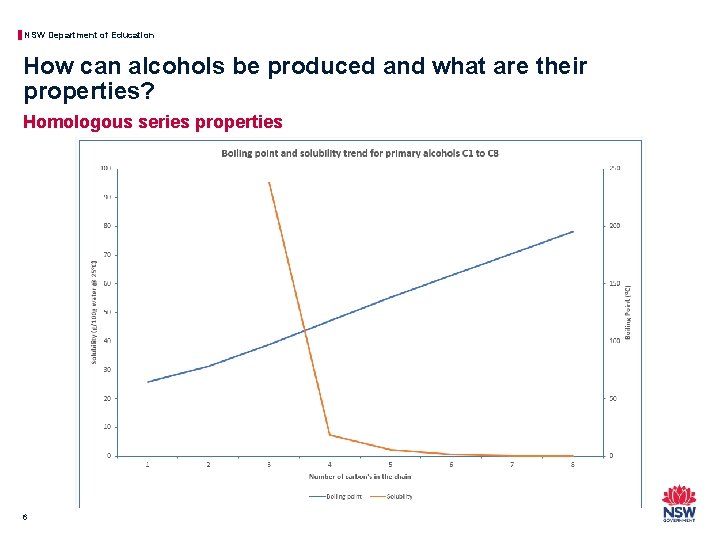
NSW Department of Education How can alcohols be produced and what are their properties? Homologous series properties 5 Name Boiling Point (o. C) Solubility (mol/100 g water @ 25 o. C) Methanol 64. 7 ∞ Ethanol 78. 3 ∞ Propan-1 -ol 97. 2 ∞ Butan-1 -ol 117. 7 0. 11 Pentan-1 -ol 138 0. 03 Hexan-1 -ol 157. 5 0. 0058 Heptan-1 -ol 176. 2 0. 0008 Octan-1 -ol 195. 2 0

NSW Department of Education How can alcohols be produced and what are their properties? Homologous series properties 6

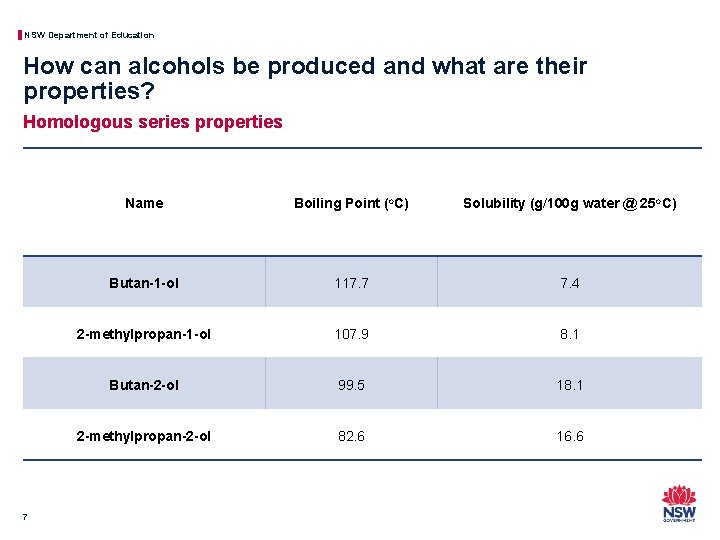
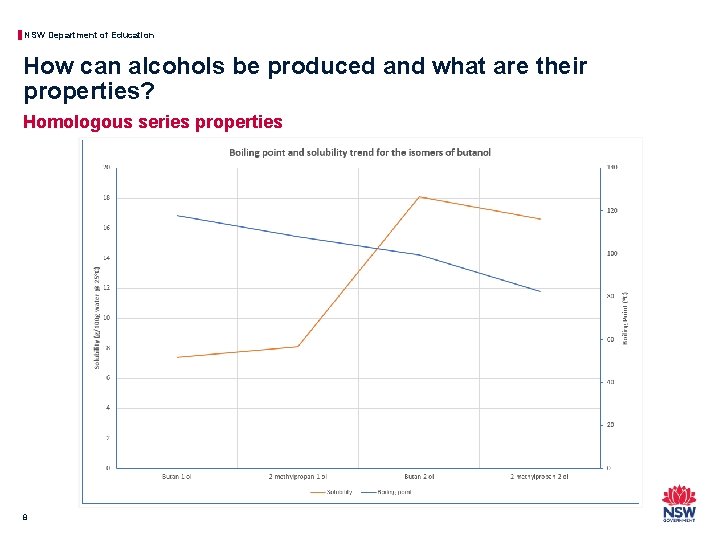
NSW Department of Education How can alcohols be produced and what are their properties? Homologous series properties 7 Name Boiling Point (o. C) Solubility (g/100 g water @ 25 o. C) Butan-1 -ol 117. 7 7. 4 2 -methylpropan-1 -ol 107. 9 8. 1 Butan-2 -ol 99. 5 18. 1 2 -methylpropan-2 -ol 82. 6 16. 6

NSW Department of Education How can alcohols be produced and what are their properties? Homologous series properties 8

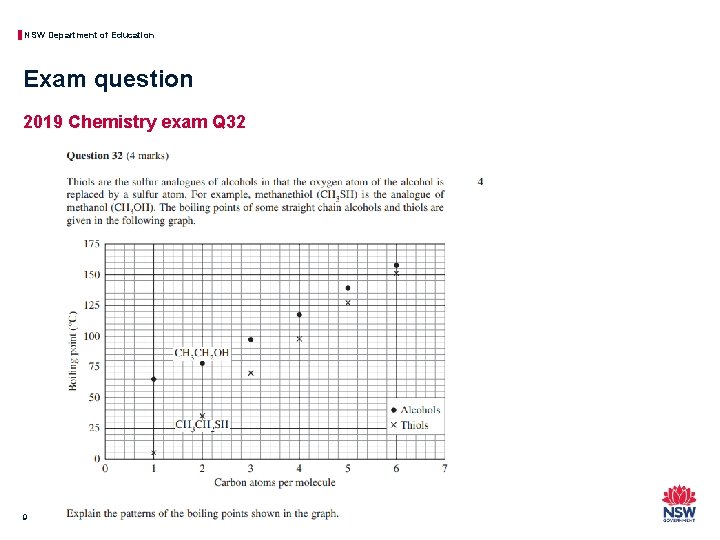
NSW Department of Education Exam question 2019 Chemistry exam Q 32 9

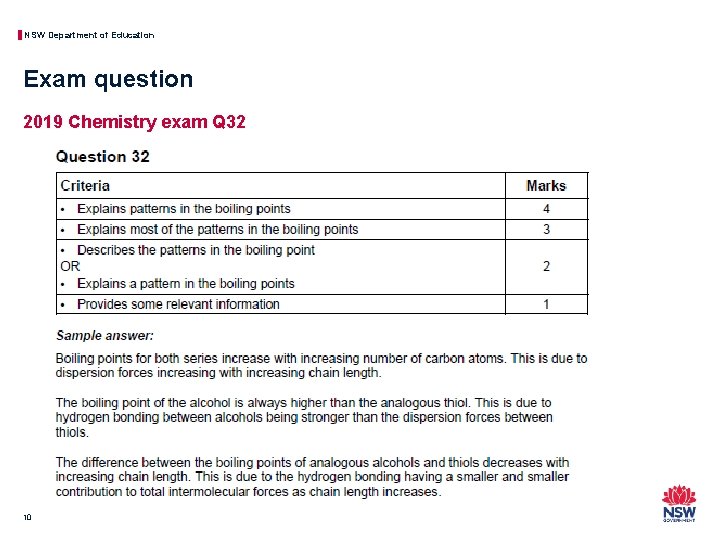
NSW Department of Education Exam question 2019 Chemistry exam Q 32 10

NSW Department of Education Exam question 2019 Chemistry exam Q 32 11

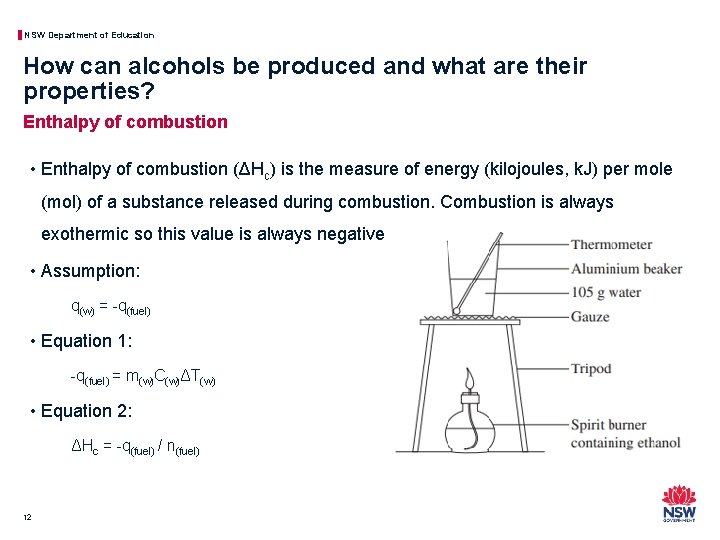
NSW Department of Education How can alcohols be produced and what are their properties? Enthalpy of combustion • Enthalpy of combustion (ΔHc) is the measure of energy (kilojoules, k. J) per mole (mol) of a substance released during combustion. Combustion is always exothermic so this value is always negative. • Assumption: q(w) = -q(fuel) • Equation 1: -q(fuel) = m(w)C(w)ΔT(w) • Equation 2: ΔHc = -q(fuel) / n(fuel) 12

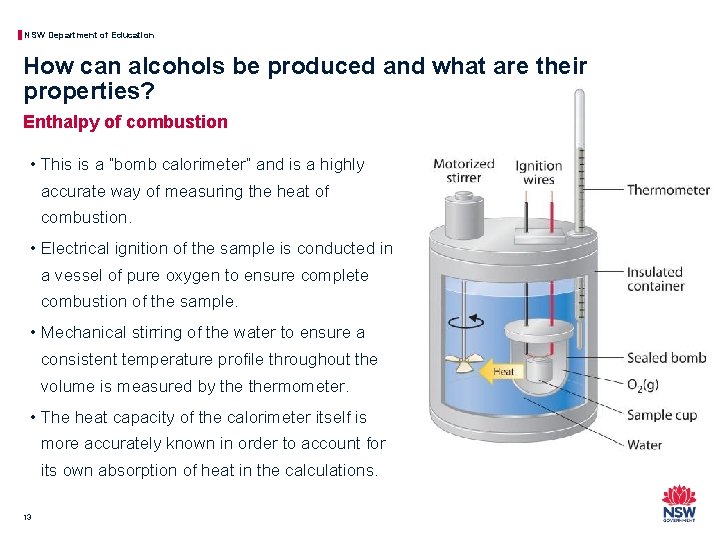
NSW Department of Education How can alcohols be produced and what are their properties? Enthalpy of combustion • This is a “bomb calorimeter” and is a highly accurate way of measuring the heat of combustion. • Electrical ignition of the sample is conducted in a vessel of pure oxygen to ensure complete combustion of the sample. • Mechanical stirring of the water to ensure a consistent temperature profile throughout the volume is measured by thermometer. • The heat capacity of the calorimeter itself is more accurately known in order to account for its own absorption of heat in the calculations. 13

NSW Department of Education How can alcohols be produced and what are their properties? Calculation 1 • Example forward calculation – 10. 0 g of ethanol is completely combusted to heat 100 g of water from 20. 0 o. C to 47. 5 o. C. Calculate ΔHc for ethanol. » Assuming q(w) = -q(fuel) » n(fuel) = m(fuel) / MW(fuel) = 10. 0 g / 46. 068 gmol-1 = 0. 217 mol ethanol combusted » ΔT(w) = (T(final) - T(initial)) = (47. 5 o. C – 20. 0 o. C) = +27. 5 temperature change of the water » m(w) = 100 g / 1000 gkg-1 = 0. 100 kg of water in the calorimeter » C(w) for water (from the Chemistry datasheet) = 4. 18 x 103 Jkg-1 K-1 » -q(fuel) = m(w)C(w)ΔT(w) = 0. 100 kg x 4. 18 x 103 Jkg-1 K-1 x 27. 5 = -11, 495 J » -q(fuel) = -11495 J / 1000 Jk. J-1 = -11. 495 k. J of energy released by the ethanol » ΔHc = -q(fuel) / n(fuel) = -11. 495 k. J / 0. 217 mol = -52. 97 k. Jmol-1 14

NSW Department of Education How can alcohols be produced and what are their properties? Calculation 2 • Example backward calculation – Propan-1 -ol is completely combusted to heat 150 g of water from 15. 0 o. C to 35. 0 o. C. Given the ΔHc = -2017. 7 k. Jmol-1 calculate the mass of propan-1 -ol which was combusted in this experiment. » Assuming q(w) = -q(fuel) » m(w) = 150 g / 1000 gkg-1 = 0. 150 kg of water in the calorimeter » ΔT(w) = (T(final) - T(initial)) = (35. 0 o. C – 15. 0 o. C) = +20. 0 temperature change of the water » -q(fuel) = m(w)C(w)ΔT(w) = 0. 150 kg x 4. 18 x 103 Jkg-1 K-1 x 20 = -12, 540 J » -q(fuel) = -12540 J / 1000 Jk. J-1 = -12. 540 k. J of energy released by the propan-1 -ol » ΔHc = -q(fuel) / n(fuel) = -q(fuel) / ΔHc = -12. 540 k. J / -2021 k. Jmol-1 = 0. 0062 mol » n(fuel) = m(fuel) / MW(fuel) m(fuel) = n(fuel) x MW(fuel) = 0. 0062 mol x 60. 094 gmol-1 = 0. 373 g 15

NSW Department of Education How can alcohols be produced and what are their properties? Enthalpy of combustion data Name 16 Enthalpy of combustion (k. Jmol-1) Methanol 726. 5 Ethanol 1367 Propan-1 -ol 2017. 7 Butan-1 -ol 2670

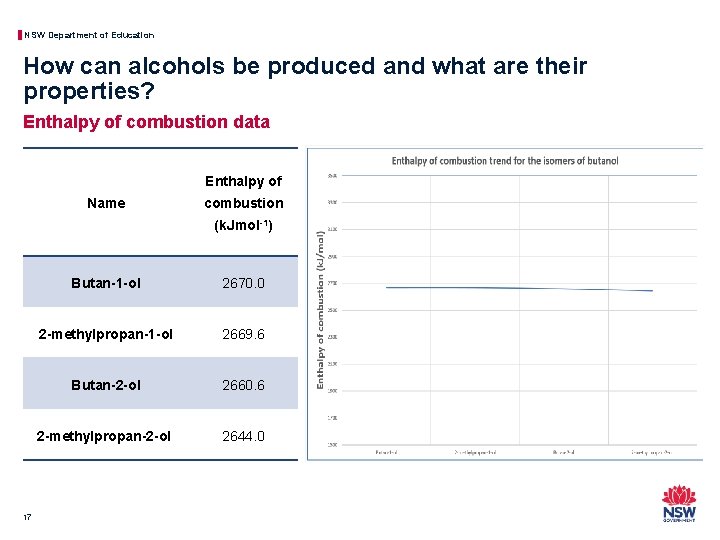
NSW Department of Education How can alcohols be produced and what are their properties? Enthalpy of combustion data Enthalpy of Name combustion (k. Jmol-1) 17 Butan-1 -ol 2670. 0 2 -methylpropan-1 -ol 2669. 6 Butan-2 -ol 2660. 6 2 -methylpropan-2 -ol 2644. 0

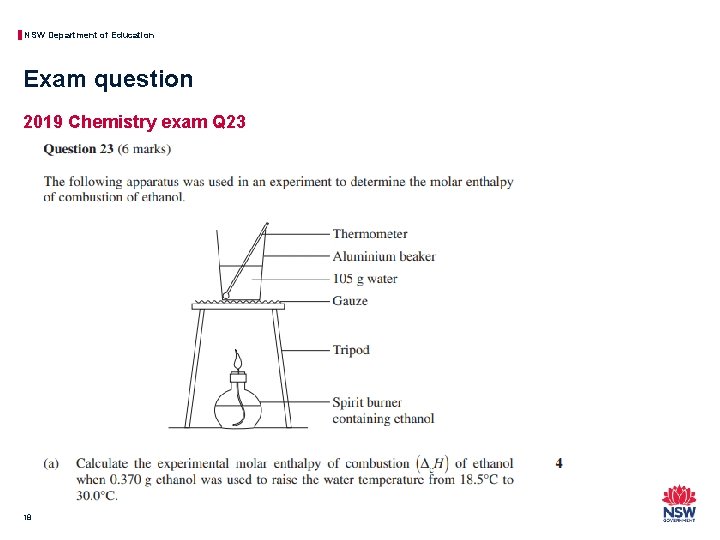
NSW Department of Education Exam question 2019 Chemistry exam Q 23 18

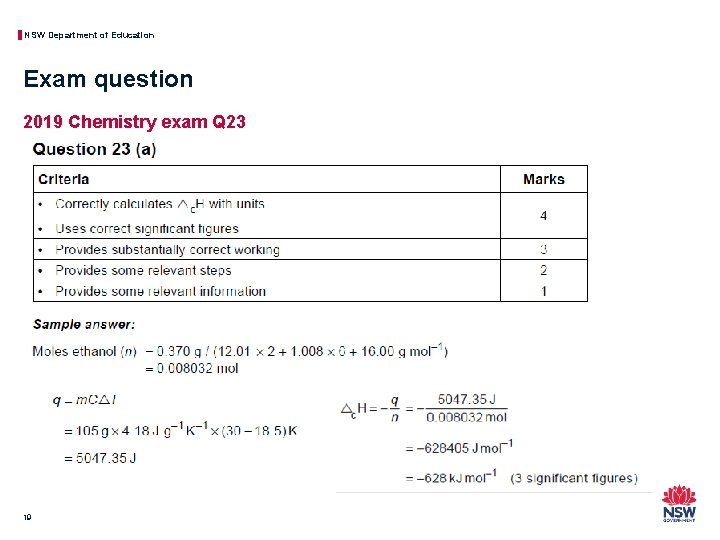
NSW Department of Education Exam question 2019 Chemistry exam Q 23 19

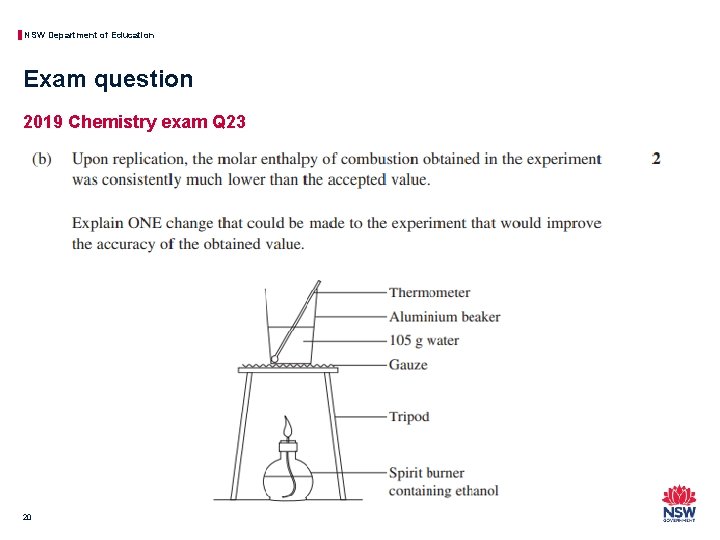
NSW Department of Education Exam question 2019 Chemistry exam Q 23 20

NSW Department of Education Exam question 2019 Chemistry exam Q 23 21

NSW Department of Education Exam question 2019 Chemistry exam Q 23 22

NSW Department of Education How can alcohols be produced and what are their properties? Combustion • Combustion is the exothermic reaction of a fuel such as alcohols with oxygen. Depending on the molar ratio of fuel to oxygen available to the reaction, two different reactions are possible: − Complete combustion occurs where oxygen is supplied in the molar ratio needed for the fuel or higher (in excess), this reaction produces only carbon dioxide and water. Complete combustion results in all bonds in the fuel being broken so maximum performance is obtained. For example, the complete combustion of ethanol: 2 C 2 H 6 O(l) + 6 O 2(g) 4 CO 2(g) + 6 H 2 O(l) » Fuel to oxygen molar ratio minimum requirement is 2: 6 = 1: 3 » Maximum energy output for this combustion is ΔHc = -1367 k. Jmol-1 23

NSW Department of Education How can alcohols be produced and what are their properties? Combustion − Incomplete combustion occurs where oxygen is supplied in a ratio less than required for the fuel to completely combust, the reaction is being starved of oxygen. This reaction can produce a wide variety of products including carbon dioxide, carbon monoxide, carbon (soot) and unburnt or partial combustion products from the fuel along with water. For example, the incomplete combustion of ethanol (there many other examples possible): 2 C 2 H 6 O(l) + 4 O 2(g) 4 CO(g) + 6 H 2 O(l) » Fuel to oxygen molar ratio is 2: 4 = 1: 2 which is less than the 1: 3 minimum requirement for complete combustion. » Maximum energy output for this combustion is less than ΔHc and is dependent on the amounts of different combustion products present. 24

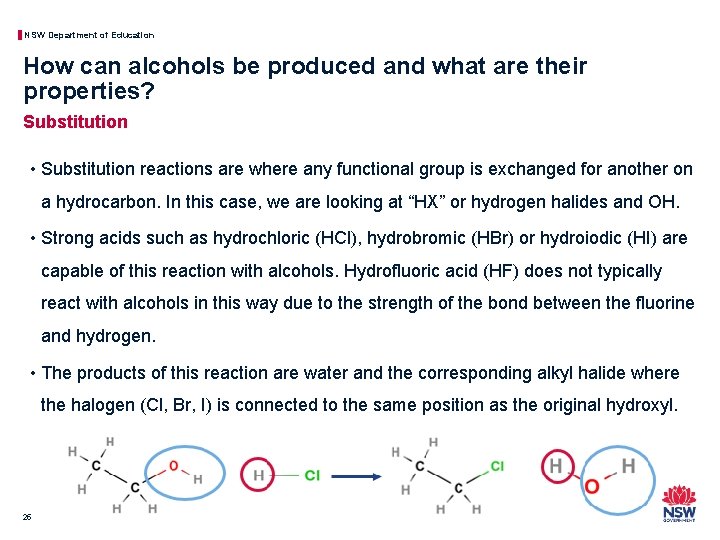
NSW Department of Education How can alcohols be produced and what are their properties? Substitution • Substitution reactions are where any functional group is exchanged for another on a hydrocarbon. In this case, we are looking at “HX” or hydrogen halides and OH. • Strong acids such as hydrochloric (HCl), hydrobromic (HBr) or hydroiodic (HI) are capable of this reaction with alcohols. Hydrofluoric acid (HF) does not typically react with alcohols in this way due to the strength of the bond between the fluorine and hydrogen. • The products of this reaction are water and the corresponding alkyl halide where the halogen (Cl, Br, I) is connected to the same position as the original hydroxyl. 25

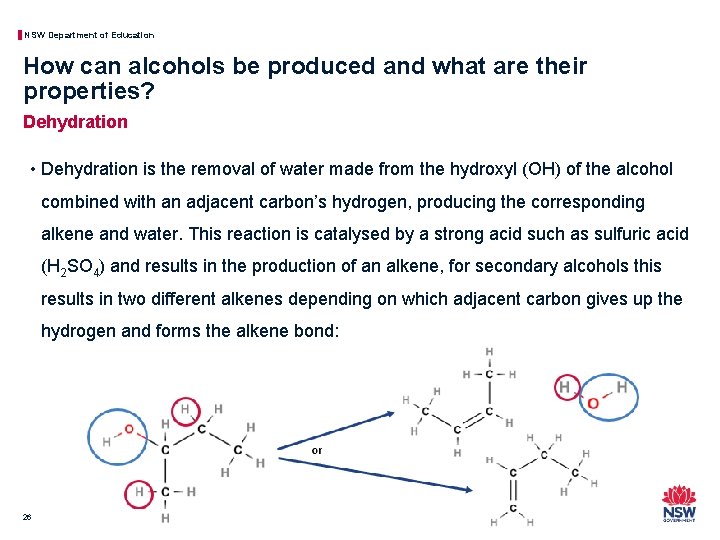
NSW Department of Education How can alcohols be produced and what are their properties? Dehydration • Dehydration is the removal of water made from the hydroxyl (OH) of the alcohol combined with an adjacent carbon’s hydrogen, producing the corresponding alkene and water. This reaction is catalysed by a strong acid such as sulfuric acid (H 2 SO 4) and results in the production of an alkene, for secondary alcohols this results in two different alkenes depending on which adjacent carbon gives up the hydrogen and forms the alkene bond: 26

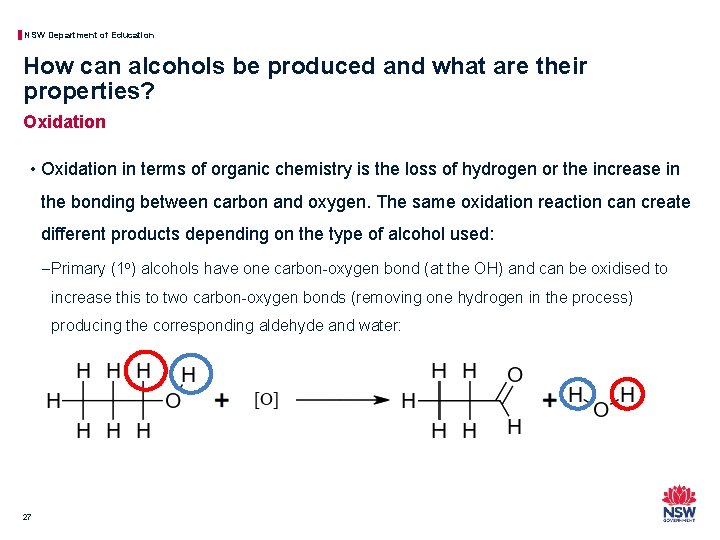
NSW Department of Education How can alcohols be produced and what are their properties? Oxidation • Oxidation in terms of organic chemistry is the loss of hydrogen or the increase in the bonding between carbon and oxygen. The same oxidation reaction can create different products depending on the type of alcohol used: − Primary (1 o) alcohols have one carbon-oxygen bond (at the OH) and can be oxidised to increase this to two carbon-oxygen bonds (removing one hydrogen in the process) producing the corresponding aldehyde and water: 27

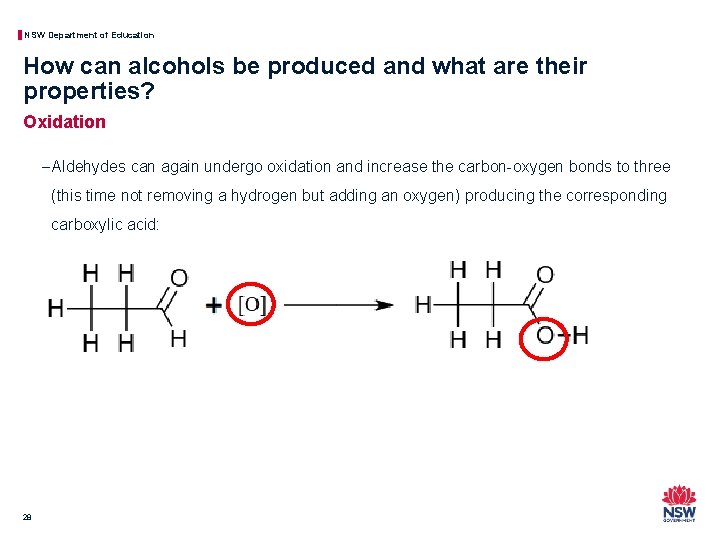
NSW Department of Education How can alcohols be produced and what are their properties? Oxidation − Aldehydes can again undergo oxidation and increase the carbon-oxygen bonds to three (this time not removing a hydrogen but adding an oxygen) producing the corresponding carboxylic acid: 28

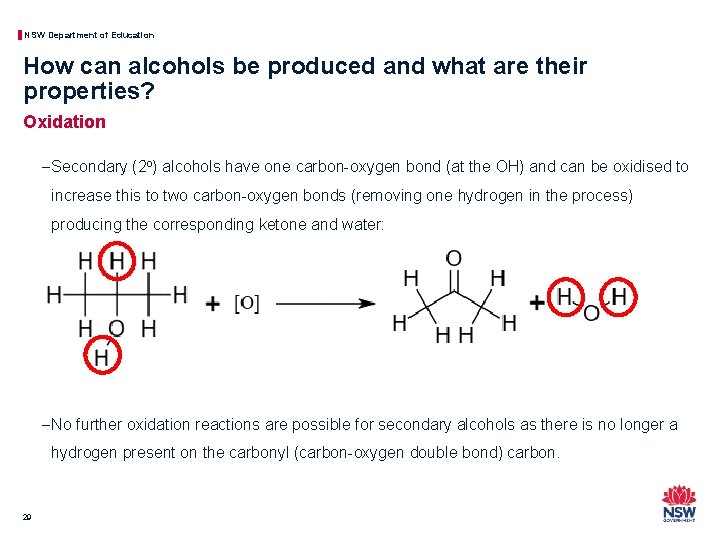
NSW Department of Education How can alcohols be produced and what are their properties? Oxidation − Secondary (2 o) alcohols have one carbon-oxygen bond (at the OH) and can be oxidised to increase this to two carbon-oxygen bonds (removing one hydrogen in the process) producing the corresponding ketone and water: − No further oxidation reactions are possible for secondary alcohols as there is no longer a hydrogen present on the carbonyl (carbon-oxygen double bond) carbon. 29

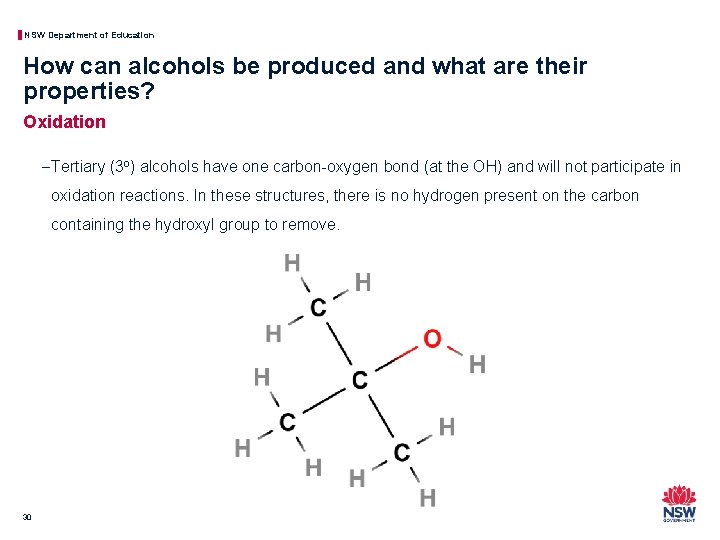
NSW Department of Education How can alcohols be produced and what are their properties? Oxidation − Tertiary (3 o) alcohols have one carbon-oxygen bond (at the OH) and will not participate in oxidation reactions. In these structures, there is no hydrogen present on the carbon containing the hydroxyl group to remove. 30

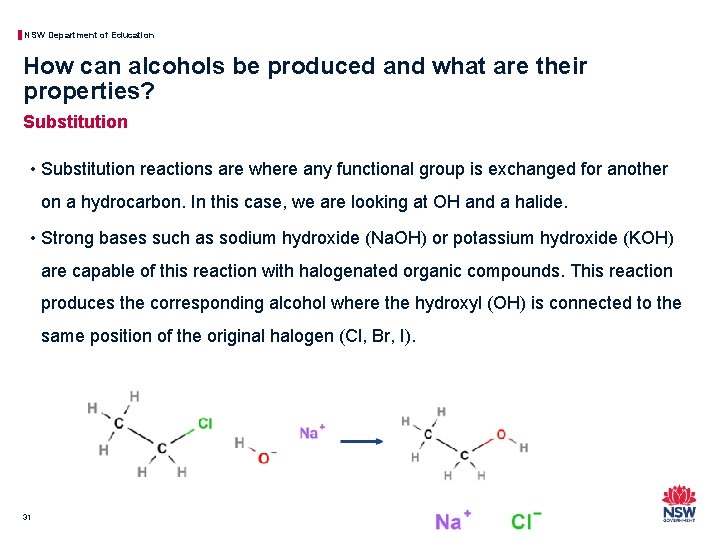
NSW Department of Education How can alcohols be produced and what are their properties? Substitution • Substitution reactions are where any functional group is exchanged for another on a hydrocarbon. In this case, we are looking at OH and a halide. • Strong bases such as sodium hydroxide (Na. OH) or potassium hydroxide (KOH) are capable of this reaction with halogenated organic compounds. This reaction produces the corresponding alcohol where the hydroxyl (OH) is connected to the same position of the original halogen (Cl, Br, I). 31

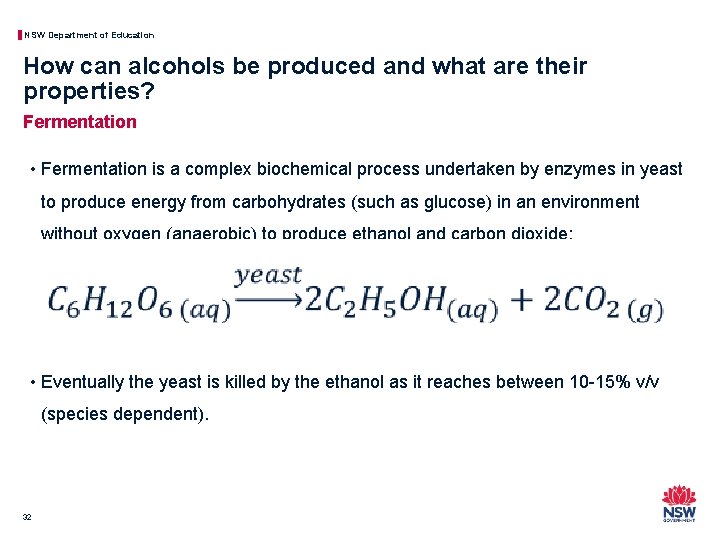
NSW Department of Education How can alcohols be produced and what are their properties? Fermentation • Fermentation is a complex biochemical process undertaken by enzymes in yeast to produce energy from carbohydrates (such as glucose) in an environment without oxygen (anaerobic) to produce ethanol and carbon dioxide: • Eventually the yeast is killed by the ethanol as it reaches between 10 -15% v/v (species dependent). 32

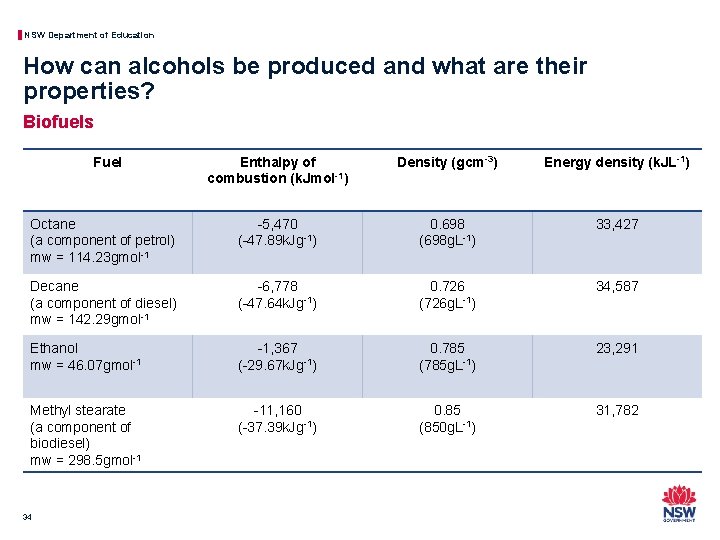
NSW Department of Education How can alcohols be produced and what are their properties? Biofuels • The term “biofuel” refers to any fuels which are produced through the use of biomass (plant or animal materials) instead of the slow geological processes that produce fossil fuels. • Comparing fuels from organic sources such as hydrocarbons in petrol and diesel to biofuels such as ethanol and biodiesel using enthalpies of combustion and energy densities are useful measures to determine the effectiveness of a fuel: − Enthalpy of combustion gives the maximum possible amount of energy per mole of the fuel completely combusted in the vehicle. − Energy density gives the amount of energy per litre of a liquid fuel. This value relates fuels to the storage requirements in the vehicle. 33

NSW Department of Education How can alcohols be produced and what are their properties? Biofuels Fuel Enthalpy of combustion (k. Jmol-1) Density (gcm-3) Energy density (k. JL-1) Octane (a component of petrol) mw = 114. 23 gmol-1 -5, 470 (-47. 89 k. Jg-1) 0. 698 (698 g. L-1) 33, 427 Decane (a component of diesel) mw = 142. 29 gmol-1 -6, 778 (-47. 64 k. Jg-1) 0. 726 (726 g. L-1) 34, 587 Ethanol mw = 46. 07 gmol-1 -1, 367 (-29. 67 k. Jg-1) 0. 785 (785 g. L-1) 23, 291 Methyl stearate (a component of biodiesel) mw = 298. 5 gmol-1 -11, 160 (-37. 39 k. Jg-1) 0. 85 (850 g. L-1) 31, 782 34
 Early childhood directorate
Early childhood directorate Nsw department of industry
Nsw department of industry Nsw health rto
Nsw health rto C device module module 1
C device module module 1 Teach lab
Teach lab Usf chemistry department
Usf chemistry department Nit calicut chemistry department
Nit calicut chemistry department Manipal university chemistry department
Manipal university chemistry department Texas tech chemistry department
Texas tech chemistry department 1st stage denial
1st stage denial Stage right vs stage left
Stage right vs stage left A stage where the audience sits on one side only
A stage where the audience sits on one side only Two stage procurement
Two stage procurement Downstage and upstage
Downstage and upstage Stage left vs stage right
Stage left vs stage right Ib organic chemistry
Ib organic chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Talent pool nsw government
Talent pool nsw government Falls risk assessment tool nsw
Falls risk assessment tool nsw Nsw energy savings scheme
Nsw energy savings scheme Fluid balance chart nsw
Fluid balance chart nsw Nsw geography syllabus
Nsw geography syllabus River systems nsw
River systems nsw Iwork nsw
Iwork nsw Andrew pedrazzini
Andrew pedrazzini Stress leave nsw
Stress leave nsw Sbir nsw
Sbir nsw Drink driving nsw facts
Drink driving nsw facts Mathematics standard stage 6 syllabus 2018
Mathematics standard stage 6 syllabus 2018 Labour hire insurance
Labour hire insurance Nsw ict services scheme
Nsw ict services scheme Plumbers direct
Plumbers direct Training services nsw
Training services nsw Pms scheme
Pms scheme Psc tracker
Psc tracker