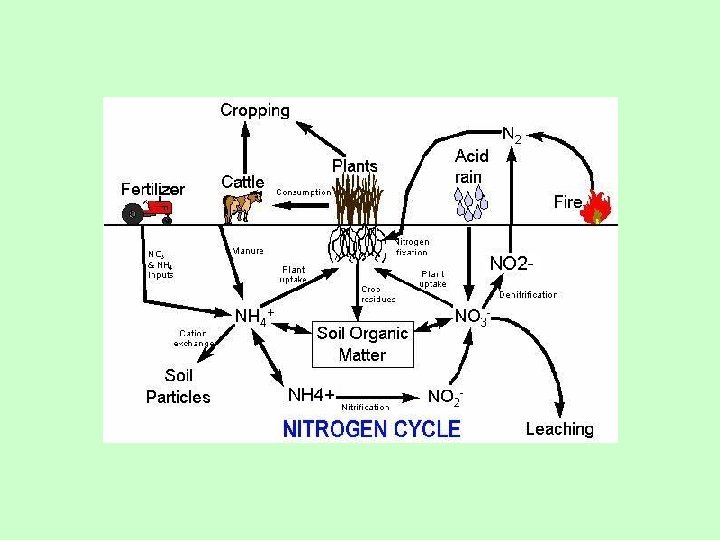
Nitrogen Cycle Sources Lightning Inorganic fertilizers Nitrogen Fixation









![Surfac e water Low [NH 4] Oxidized layer Reduce d soil layer Slow Diffusion Surfac e water Low [NH 4] Oxidized layer Reduce d soil layer Slow Diffusion](https://slidetodoc.com/presentation_image_h2/1c6af46212bacad878e88e1799bc166e/image-28.jpg)
![Surfac e water nitrificatio n Low [NH 4] Oxidized layer Reduce d soil layer Surfac e water nitrificatio n Low [NH 4] Oxidized layer Reduce d soil layer](https://slidetodoc.com/presentation_image_h2/1c6af46212bacad878e88e1799bc166e/image-29.jpg)
![N 2 Surfac e water Oxidized layer Reduce d soil layer [NO 3] high N 2 Surfac e water Oxidized layer Reduce d soil layer [NO 3] high](https://slidetodoc.com/presentation_image_h2/1c6af46212bacad878e88e1799bc166e/image-30.jpg)

- Slides: 31

Nitrogen Cycle

Sources • • • Lightning Inorganic fertilizers Nitrogen Fixation Animal Residues Crop residues Organic fertilizers


Forms of Nitrogen • • Urea CO(NH 2)2 Ammonia NH 3 (gaseous) Ammonium NH 4 Nitrate NO 3 Nitrite NO 2 Atmospheric Dinitrogen N 2 Organic N

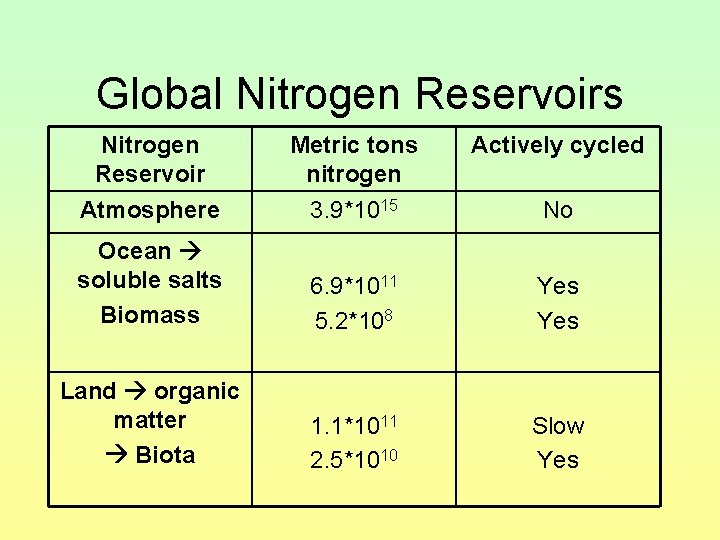
Global Nitrogen Reservoirs Nitrogen Reservoir Metric tons nitrogen Actively cycled Atmosphere 3. 9*1015 No Ocean soluble salts Biomass 6. 9*1011 5. 2*108 Yes Land organic matter Biota 1. 1*1011 2. 5*1010 Slow Yes

Roles of Nitrogen • Plants and bacteria use nitrogen in the form of NH 4+ or NO 3 • It serves as an electron acceptor in anaerobic environment • Nitrogen is often the most limiting nutrient in soil and water.

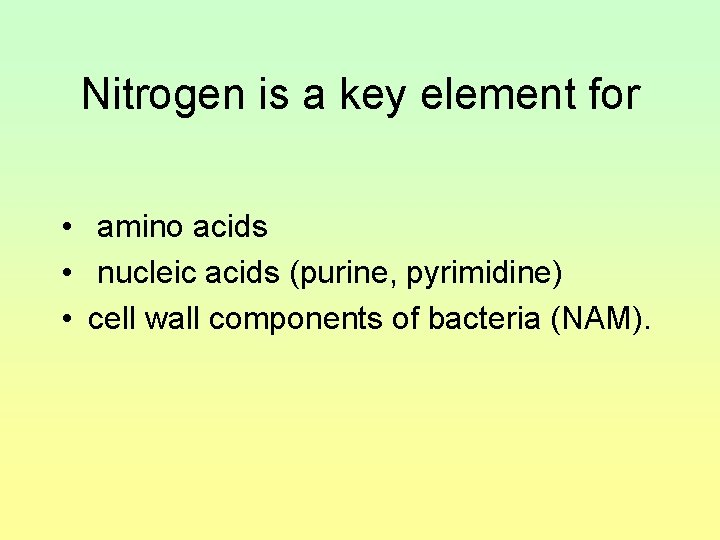
Nitrogen is a key element for • amino acids • nucleic acids (purine, pyrimidine) • cell wall components of bacteria (NAM).

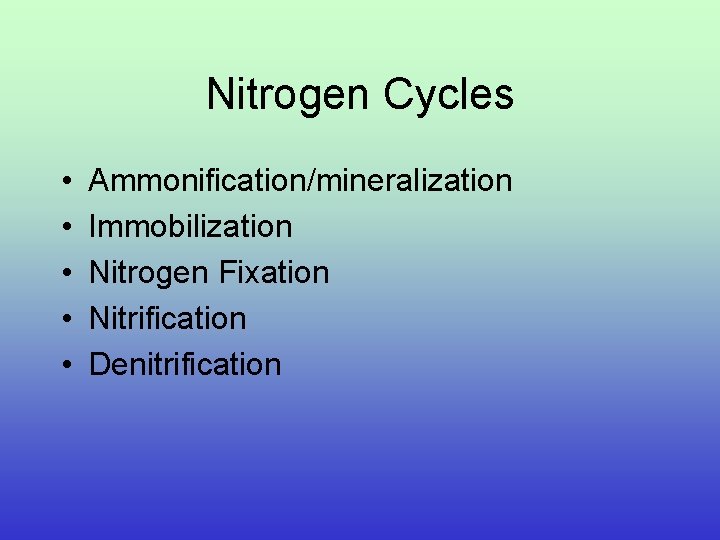
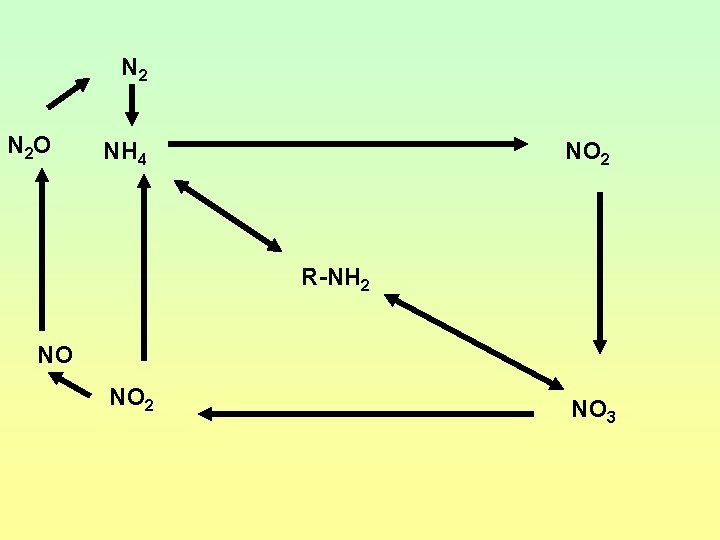
Nitrogen Cycles • • • Ammonification/mineralization Immobilization Nitrogen Fixation Nitrification Denitrification

N 2 N 2 O NH 4 NO 2 R-NH 2 NO NO 2 NO 3

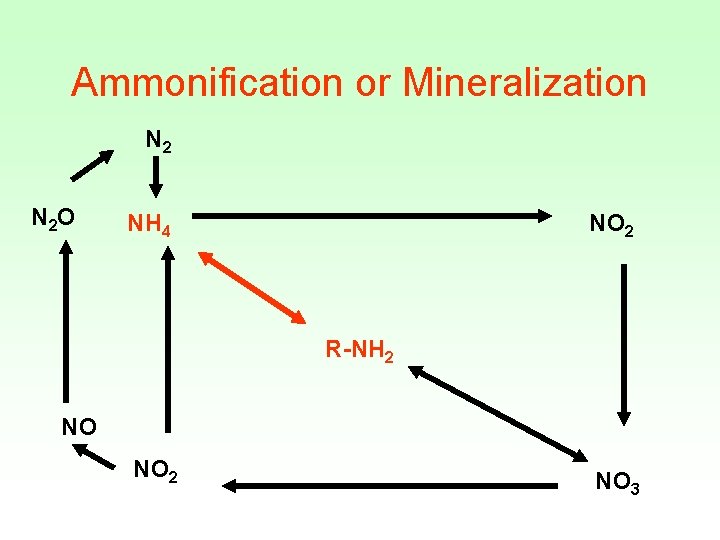
Ammonification or Mineralization N 2 O NH 4 NO 2 R-NH 2 NO NO 2 NO 3

Mineralization or Ammonification • Decomposers: earthworms, termites, slugs, snails, bacteria, and fungi • Uses extracellular enzymes initiate degradation of plant polymers • Microorganisms uses: • Proteases, lysozymes, nucleases to degrade nitrogen containing molecules

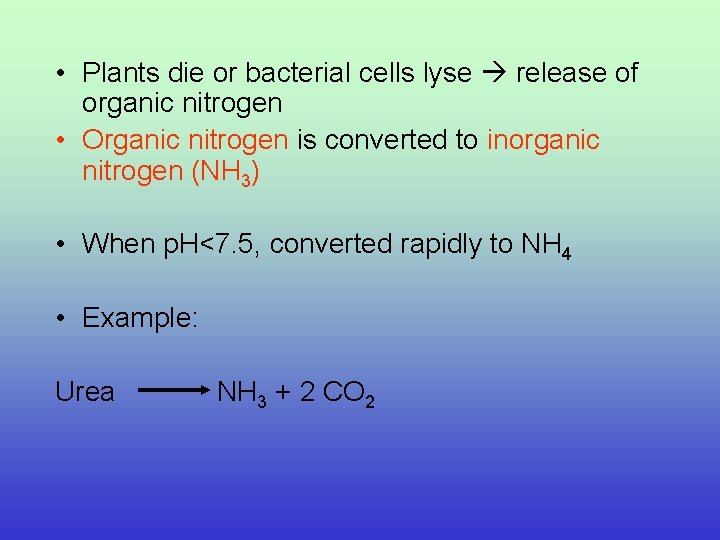
• Plants die or bacterial cells lyse release of organic nitrogen • Organic nitrogen is converted to inorganic nitrogen (NH 3) • When p. H<7. 5, converted rapidly to NH 4 • Example: Urea NH 3 + 2 CO 2

Immobilization • The opposite of mineralization • Happens when nitrogen is limiting in the environment • Nitrogen limitation is governed by C/N ratio • C/N typical for soil microbial biomass is 20 • C/N < 20 Mineralization • C/N > 20 Immobilization

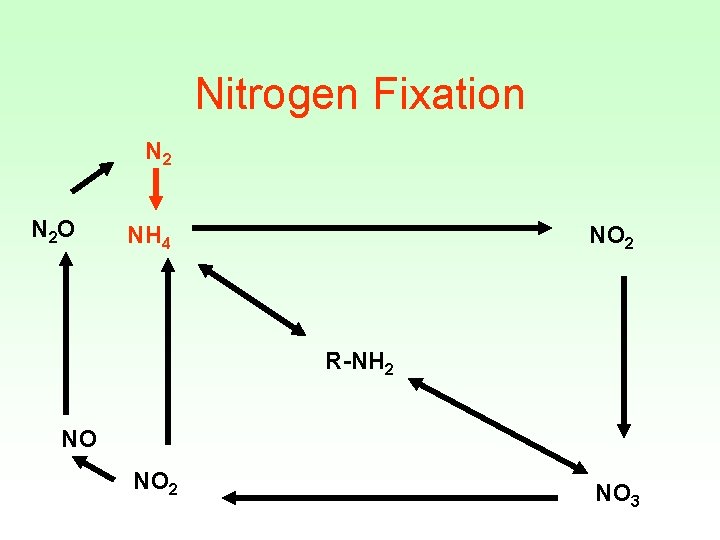
Nitrogen Fixation N 2 O NH 4 NO 2 R-NH 2 NO NO 2 NO 3

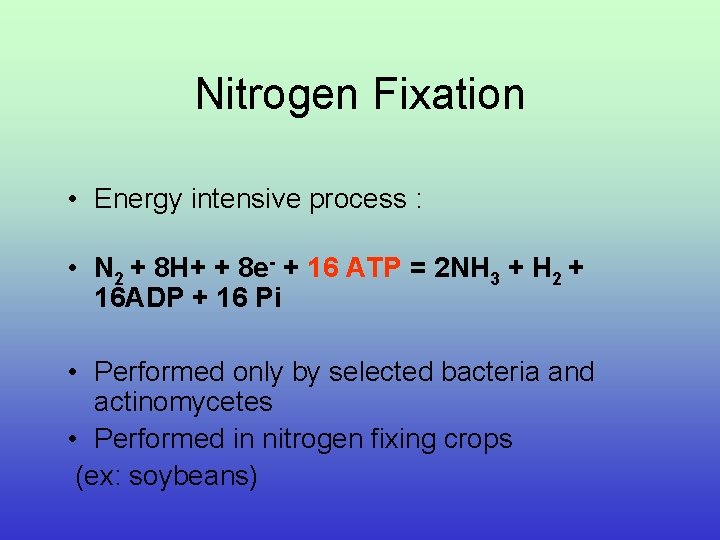
Nitrogen Fixation • Energy intensive process : • N 2 + 8 H+ + 8 e- + 16 ATP = 2 NH 3 + H 2 + 16 ADP + 16 Pi • Performed only by selected bacteria and actinomycetes • Performed in nitrogen fixing crops (ex: soybeans)

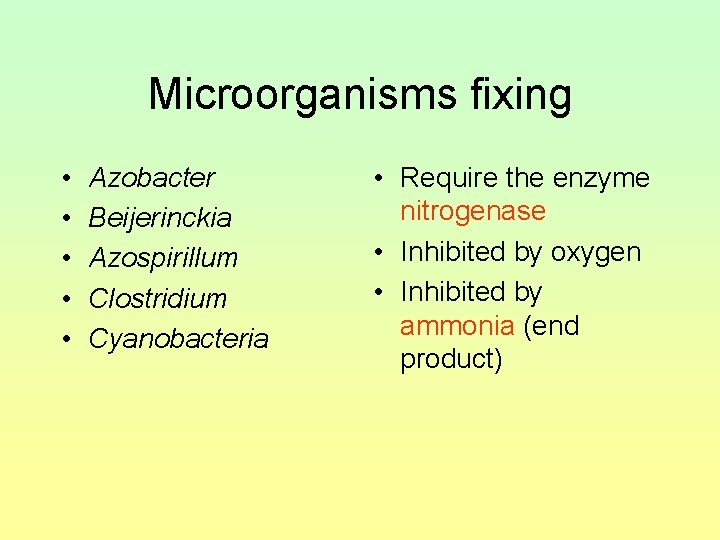
Microorganisms fixing • • • Azobacter Beijerinckia Azospirillum Clostridium Cyanobacteria • Require the enzyme nitrogenase • Inhibited by oxygen • Inhibited by ammonia (end product)

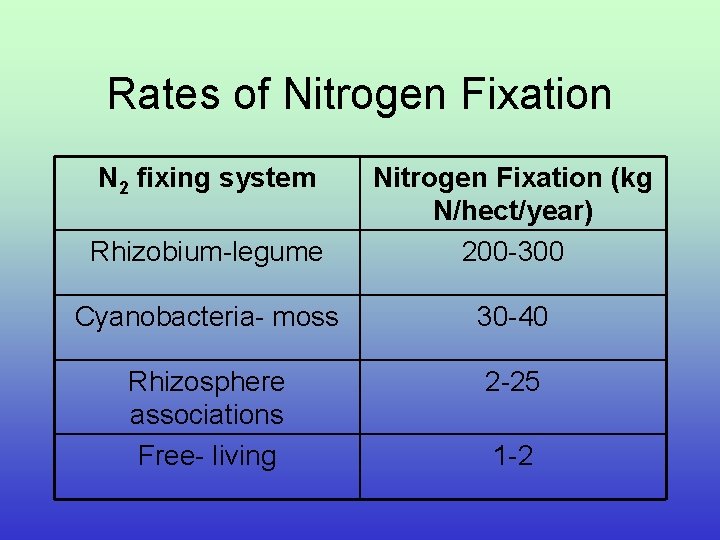
Rates of Nitrogen Fixation N 2 fixing system Rhizobium-legume Nitrogen Fixation (kg N/hect/year) 200 -300 Cyanobacteria- moss 30 -40 Rhizosphere associations Free- living 2 -25 1 -2


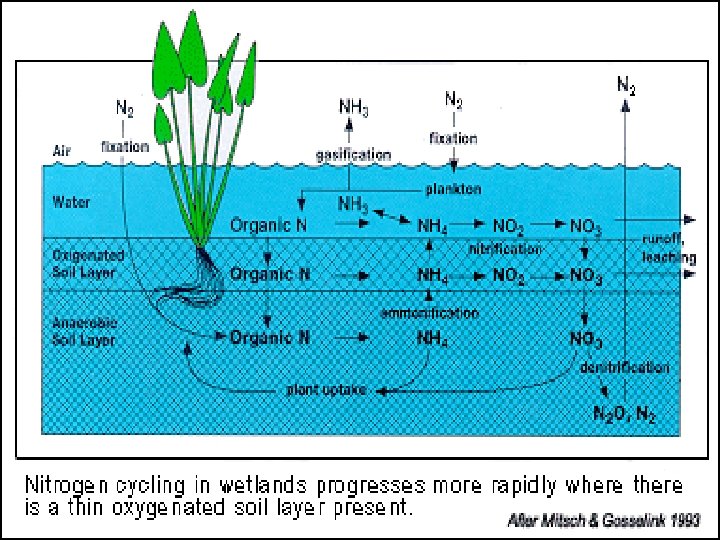
Applications to wetlands • • • Occur in overlying waters Aerobic soil Anaerobic soil Oxidized rhizosphere Leaf or stem surfaces of plants

Bacterial Fixation • Occurs mostly in salt marshes • Is absent from low p. H peat of northern bogs • Cyanobacteria found in waterlogged soils

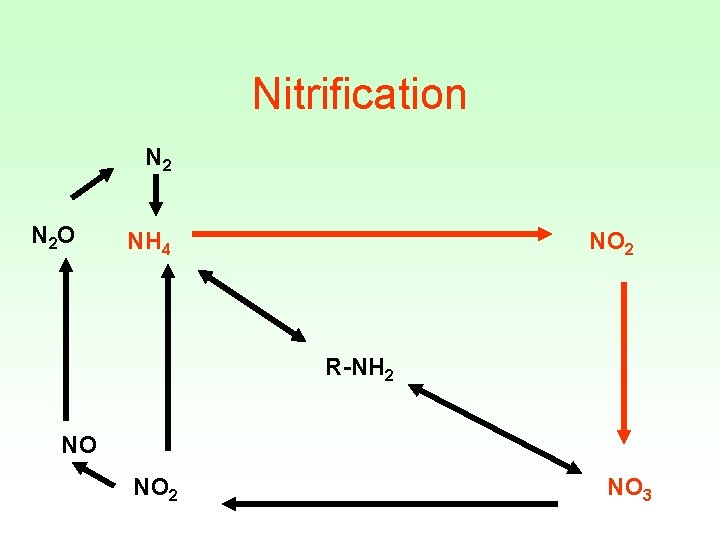
Nitrification N 2 O NH 4 NO 2 R-NH 2 NO NO 2 NO 3

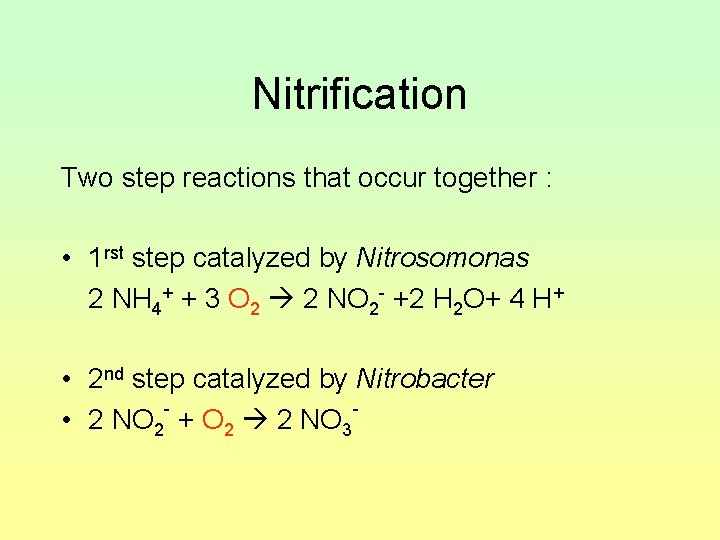
Nitrification Two step reactions that occur together : • 1 rst step catalyzed by Nitrosomonas 2 NH 4+ + 3 O 2 2 NO 2 - +2 H 2 O+ 4 H+ • 2 nd step catalyzed by Nitrobacter • 2 NO 2 + O 2 2 NO 3

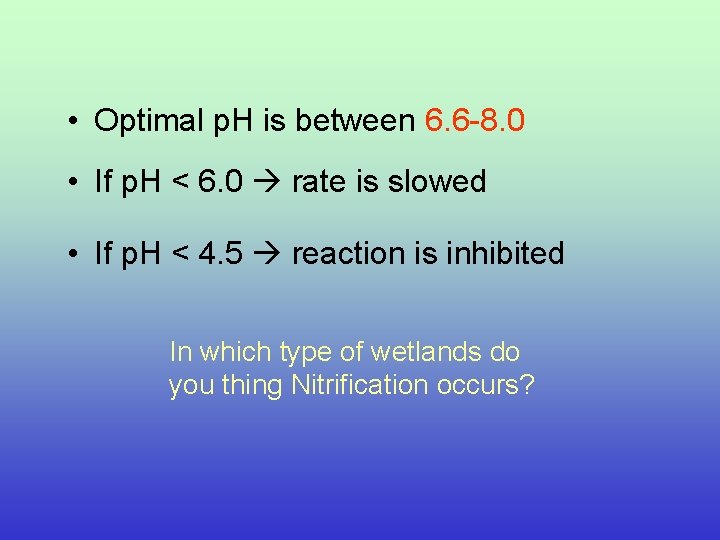
• Optimal p. H is between 6. 6 -8. 0 • If p. H < 6. 0 rate is slowed • If p. H < 4. 5 reaction is inhibited In which type of wetlands do you thing Nitrification occurs?

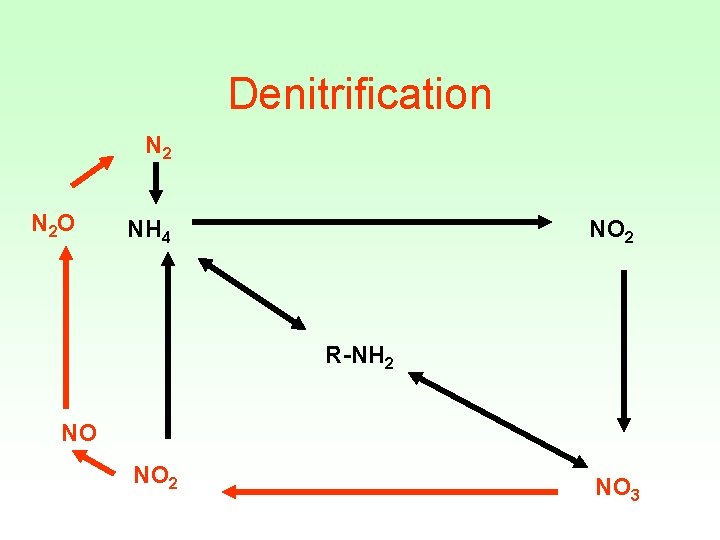
Denitrification N 2 O NH 4 NO 2 R-NH 2 NO NO 2 NO 3

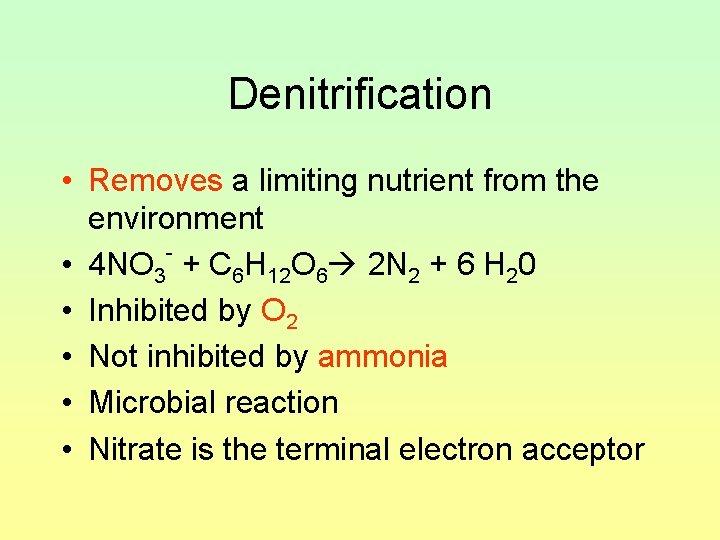
Denitrification • Removes a limiting nutrient from the environment • 4 NO 3 + C 6 H 12 O 6 2 N 2 + 6 H 20 • Inhibited by O 2 • Not inhibited by ammonia • Microbial reaction • Nitrate is the terminal electron acceptor

Looking at the Nitrogen cycle through the eye of NH 4

![Surfac e water Low NH 4 Oxidized layer Reduce d soil layer Slow Diffusion Surfac e water Low [NH 4] Oxidized layer Reduce d soil layer Slow Diffusion](https://slidetodoc.com/presentation_image_h2/1c6af46212bacad878e88e1799bc166e/image-28.jpg)
Surfac e water Low [NH 4] Oxidized layer Reduce d soil layer Slow Diffusion [NH 4] HIGH Biodegradati on C/N <20 C/N >20
![Surfac e water nitrificatio n Low NH 4 Oxidized layer Reduce d soil layer Surfac e water nitrificatio n Low [NH 4] Oxidized layer Reduce d soil layer](https://slidetodoc.com/presentation_image_h2/1c6af46212bacad878e88e1799bc166e/image-29.jpg)
Surfac e water nitrificatio n Low [NH 4] Oxidized layer Reduce d soil layer Slow Diffusion [NH 4] HIGH [NO 3] high
![N 2 Surfac e water Oxidized layer Reduce d soil layer NO 3 high N 2 Surfac e water Oxidized layer Reduce d soil layer [NO 3] high](https://slidetodoc.com/presentation_image_h2/1c6af46212bacad878e88e1799bc166e/image-30.jpg)
N 2 Surfac e water Oxidized layer Reduce d soil layer [NO 3] high Leaching [NO 3] Low Denitrificatio n
