NITRATE AND NITRITE VPP 609 Toxicopathology Unit II
















- Slides: 20

NITRATE AND NITRITE VPP 609 Toxicopathology Unit II Dr. Sanjiv Kumar, Assistant Professor, Department of Veterinary Pathology, BVC, Patna

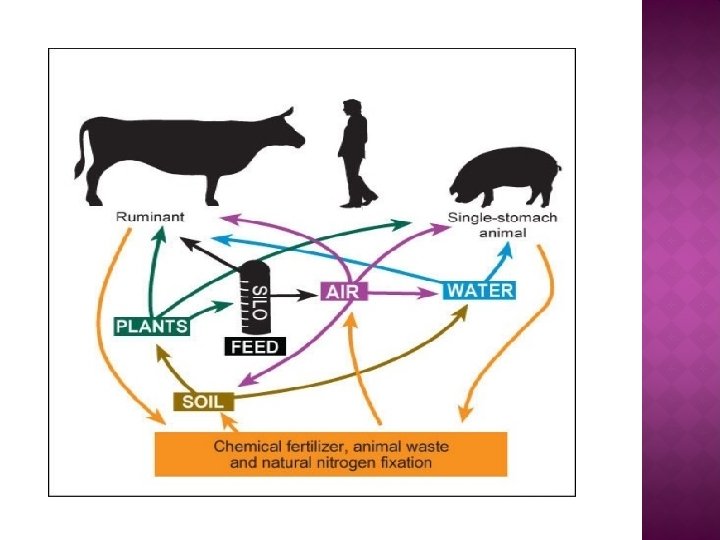
Nitrate itself is not toxic to animals, but at elevated levels, it can cause a noninfectious disease called nitrite poisoning. Nitrate poisoning is generally caused when animals eat too much forage that is high in nitrates not changed to protein in the plant. Poisoning can also happen when animals eat too much urea or nitrogen fertilizer spilled in the field or left where the animal scan find it.

Normally Nitrate is broken down to nitrite (NO 2) and then to ammonia (NH 3), Ammonia is then converted to protein by microbes in the rumen (which is the compartment of the ruminant stomach). If Nitrite is absorbed into the blood and combines with hemoglobin to form met-hemoglobin, which causes a reduction in the ability of the blood to carry oxygen from the lungs to body tissues.

All plants contain some nitrate, but excessively high levels are likely to occur in forages grown under stress conditions. � Nitrates accumulate in plants only when: i. A large amount of nitrate in the soil. ii. Some factor interferes with normal plant growth. � Used in pickling and curing brines for preserving meats, certain machine oils and antirust tablets, gunpowder and explosives, and fertilizers. Nitrate toxicosis can also result from accidental ingestion of fertilizer or other chemicals. Nitrate concentrations may be hazardous in ponds that receive extensive feedlot or fertilizer runoff; these types of nitrate sources may also contaminate shallow, poorly cased wells.

� Plants convert nitrate (NO 3) to nitrite (NO 2) which in turn is converted to ammonia and then to amino acids, the building blocks of protein. � During the night, nitrate accumulates when photosynthesis is inactive, then during the day, nitrate is quickly converted to protein. � The highest levels of nitrate accumulate when drought occurs during heavy nitrate uptake by the plant.


Normally nitrate is assimilated so rapidly following absorption from soil that its concentration in plant tissues is low. � The most notorious accumulators of nitrate are the sorghums. Other annuals that less frequently accumulate nitrate are small grains. � Some perennial grasses and weeds ( mustard, nightshade and lamb’s quarters) also can contain dangerous levels. The corn may be safe but weeds harvested with it may be poisonous. � Accumulation usually is triggered by some environmental stress where plant growth is restricted but absorption of nitrate from soil continues. � Environment factors includes reduce sunlight, frost, certain herbicides, acid soils, low growing temperatures, and deficiencies of essential nutrients like phosphorus, sulfur and molybdenum. �


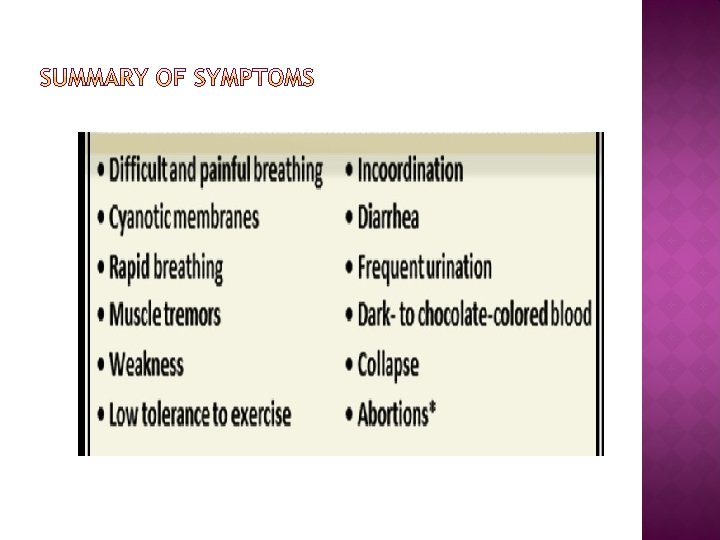
� � � Signs of nitrite poisoning usually appear suddenly due to tissue hypoxia and low blood pressure as a consequence of vasodilation. The nitrite ion may also alter metabolic protein enzymes. Ingested nitrates may directly irritate the GI mucosa and produce abdominal pain and diarrhea. The first symptom to appear is a grayish to brownish discoloration of non pigmented skin and mucous membranes of the mouth, nose, eyes and vulva. This discoloration results from the chocolate-brown color of the blood, a distinct characteristic of nitrate toxicity that persists several hours after death. As the syndrome progresses, a staggering gait, rapid pulse, labored breathing and frequent urination develop, followed by collapse, coma and death. Symptoms often occur rapidly, within one-half to four hours after ingestion of a toxic dose. Some animals exhibit symptoms but recover spontaneously and completely.



� Blood that contains methemoglobin usually has a chocolate-brown color, although dark red hues may also be seen. � There may be pinpoint or larger hemorrhages on serosal surfaces. � Dark brown discoloration evident in moribund or recently dead animals is not pathognomonic, however, and other methemoglobin inducers must be considered. � If necropsy is postponed too long, the brown discoloration may disappear with conversion of methemoglobin back to Hgb.




� Observed clinical signs. � Possible exposure to toxic plants, feeds or water. � Post-mortem findings. � Laboratory tests.


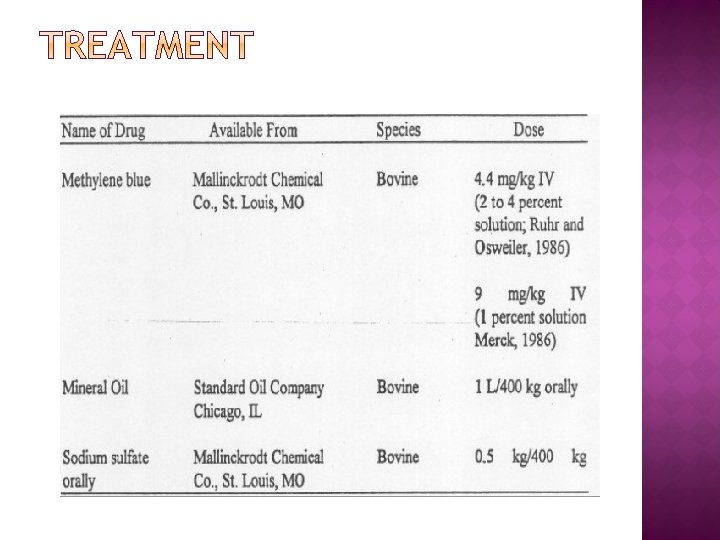
� Urgent veterinary attention is required to confirm the tentative diagnosis and to treat affected animals. � Stock should immediately be removed from suspect material, and be handled as little and as quietly as possible. � Hay or some other low-nitrate herbage should be fed to dilute the nitrate and/or nitrite in the stomach. � Affected animals can be treated by intravenous injections of methylene blue, a powdered dye material. Methylene blue converts the methaemoglobin back to oxygen- carrying hemoglobin.

� Death loss from nitrate is an occasional problem in cattle consuming certain annual forages, particularly sorghum hybrids. � High nitrate forages can be used if diluted with other feedstuffs and supplemented with energy. � Nitrate toxicity is sometimes a lethal problem for livestock especially during the fall. � Avoid poisoning with good management practices. � A qualitative check called the diphenylamine test can be used to screen forages for potential harm.

THANK YOU