Next Gen Studies Active and Planned Self expanding


















- Slides: 28

Next Gen Studies (Active and Planned) Self expanding valves Michael J. Reardon, M. D. Professor of Cardiothoracic Surgery Allison Family Distinguished Chair of Cardiovascular Research Houston Methodist Hospital

Michael J. Reardon, M. D. Advisory Board/ consultant Medtronic, Boston Scientific, Gore Medical

Self expanding valves Evolut R/Pro Plus Portico/ Portico Flex Nav/Navitor Acurate neo 2 Jena Valve J Valve




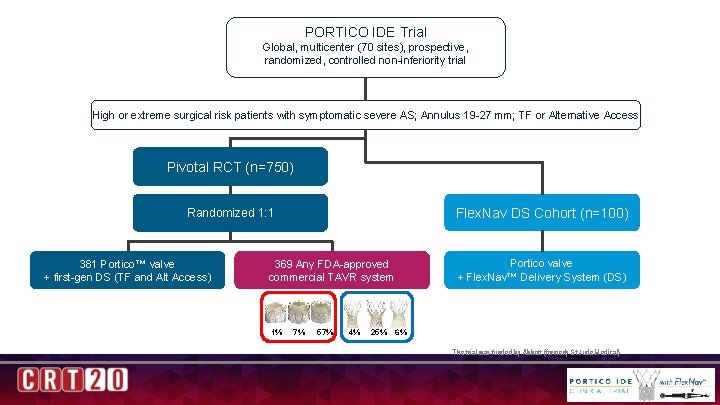
PORTICO IDE Trial Design Global, multicenter (70 sites), prospective, randomized, controlled non-inferiority trial High or extreme surgical risk patients with symptomatic severe AS; Annulus 19 -27 mm; TF or Alternative Access Pivotal RCT (n=750) Flex. Nav DS Cohort (n=100) Randomized 1: 1 381 Portico™ valve + first-gen DS (TF and Alt Access) Portico valve + Flex. Nav™ Delivery System (DS) 369 Any FDA-approved commercial TAVR system 1% 7% 57% 4% 25% 6% The trial was funded by Abbott (formerly St Jude Medical)

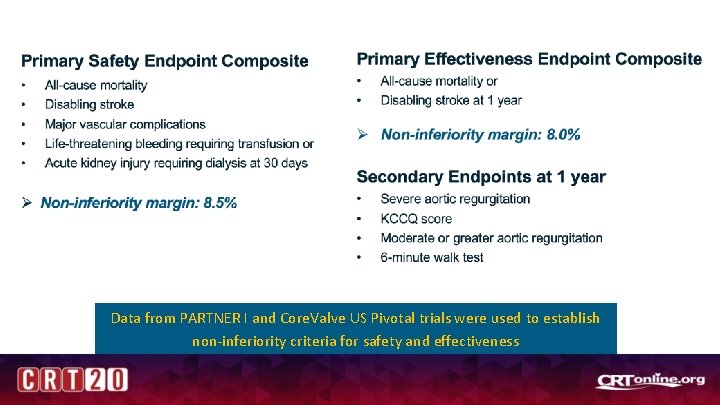
Data from PARTNER I and Core. Valve US Pivotal trials were used to establish non-inferiority criteria for safety and effectiveness

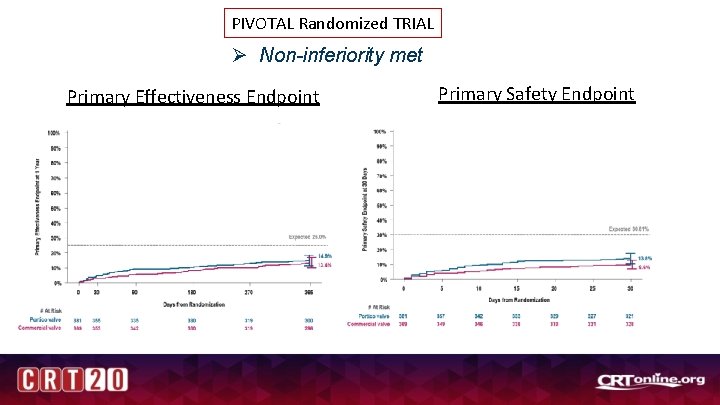
PIVOTAL Randomized TRIAL Ø Non-inferiority met Primary Effectiveness Endpoint Author (s) Affiliation On behalf of. . . (Delete if N/A) Primary Safety Endpoint

Portico™ vs Flex. Nav™ Delivery System • • • 14 -15 French equivalent Stability layer for accurate placement Integrated sheath Hydrophilic coating Redesigned handle

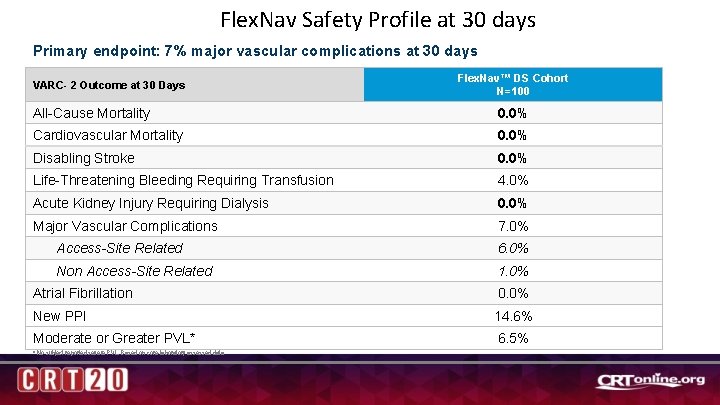
Flex. Nav Safety Profile at 30 days Primary endpoint: 7% major vascular complications at 30 days VARC- 2 Outcome at 30 Days Flex. Nav™ DS Cohort N=100 All-Cause Mortality 0. 0% Cardiovascular Mortality 0. 0% Disabling Stroke 0. 0% Life-Threatening Bleeding Requiring Transfusion 4. 0% Acute Kidney Injury Requiring Dialysis 0. 0% Major Vascular Complications 7. 0% Access-Site Related 6. 0% Non Access-Site Related 1. 0% Atrial Fibrillation 0. 0% New PPI 14. 6% Moderate or Greater PVL* 6. 5% * No subject reported severe PVL. Based on core-laboratory assessed data

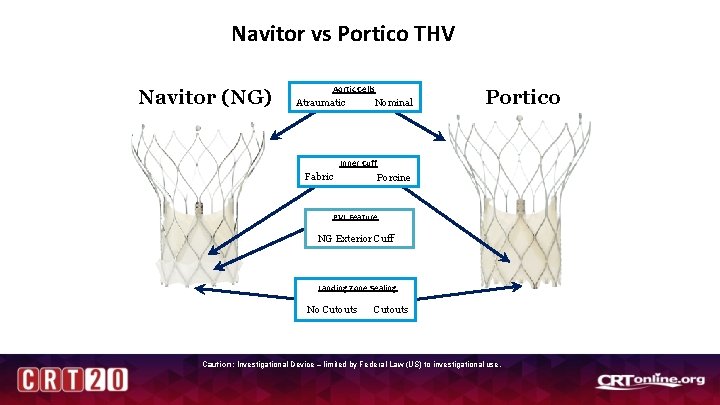
Navitor vs Portico THV Navitor (NG) Aortic Cells Atraumatic Nominal Portico Inner Cuff Fabric Porcine PVL Feature NG Exterior Cuff Landing Zone Sealing No Cutouts Caution: Investigational Device – limited by Federal Law (US) to investigational use.

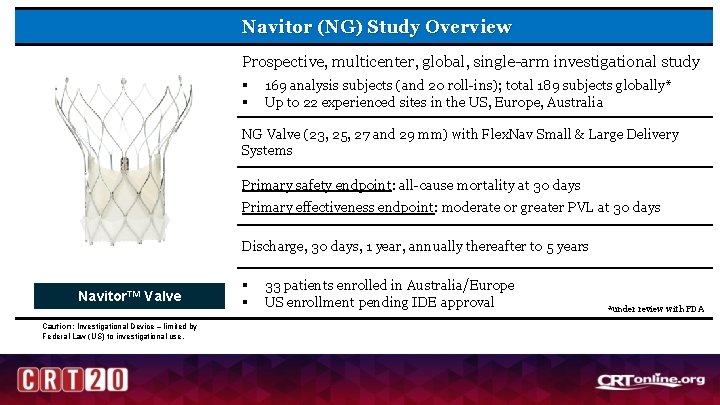
Navitor (NG) Study Overview Prospective, multicenter, global, single-arm investigational study § § 169 analysis subjects (and 20 roll-ins); total 189 subjects globally* Up to 22 experienced sites in the US, Europe, Australia NG Valve (23, 25, 27 and 29 mm) with Flex. Nav Small & Large Delivery Systems Primary safety endpoint: all-cause mortality at 30 days Primary effectiveness endpoint: moderate or greater PVL at 30 days Discharge, 30 days, 1 year, annually thereafter to 5 years Navitor™ Valve Caution: Investigational Device – limited by Federal Law (US) to investigational use. § § 33 patients enrolled in Australia/Europe US enrollment pending IDE approval *under review with FDA

Participating Sites Site Name (US) Co-Study PIs: Dr. Michael Reardon Prof. Lars Søndergaard Principal Investigator Advocate Christ Medical Center Ravi Ramana Baptist Memorial Hospital Edward Garrett Cardiovascular Research Institute of Kansas Bassem Chehab Cedars-Sinai Medical Center Raj Makkar Los Robles Regional Medical Center Gregory Fontana Principal Investigator Minneapolis Heart Institute Paul Sorajja Fiona Stanley Hospital Gerald Yong Mission Health & Hospitals Mark Groh The Alfred Hospital Tony Walton Susheel Kodali St. Andrew’s Hospital/Wesley Stephen Worthley New York-Presbyterian/Columbia University Medical Center Rigshospitalet Lars Søndergaard Sparrow Clinical Research Institute Gaurav Dhar Policlinico San Donato Francesco Bedogni St. Vincent Hospital James Hermiller Royal Victoria Hospital Ganesh Manoharan The Heart Hospital Baylor Plano Katherine Harrington Houston Methodist Hospital Neal Kleiman Washington Hospital Center Ron Waksman Site Name (EU/AUS) *currently enrolling sites

Key Inclusion/Exclusion Criteria Inclusion Exclusion • STS score of ≥ 7% OR documented heart team • Need for emergency surgery agreement of high or extreme risk for surgical • Acute MI within 30 days prior to index aortic valve replacement procedure • NYHA II or greater • Stroke or TIA within 6 months of the index • AVA ≤ 1. 0 cm 2 or indexed EOA ≤ 0. 6 cm 2/m 2 • Mean gradient ≥ 40 mm. Hg or peak jet velocity ≥ 4. 0 m/s or doppler velocity index (DVI) ≤ 0. 25 • Aortic annulus diameter of 19‐ 27 mm and ascending aorta diameter of 26 -42 mm procedure • Mixed aortic valve disease • Unicuspid or bicuspid aortic valve • LVEF <25% • Severe mitral or tricuspid regurgitation or severe right ventricle dysfunction

• DESIGN GOALS – – – – Active Outer Cuff PVL-Sealing Feature 1 Optimized Radial Force 2 Curved Aortic Frame Section 1 Lower Profile Inner Sealing Cuff 1 Patient annulus range 19 -27 mm 1 Large Cell Geometry & Intra-Annular Valve Position 2 Early Valve Functionality, No Rapid Pacing 2 Recapturable, Repositionable, Retrievable 2 NAVITOR™ Valve 1. Data on file at Abbott. Report WC#600081963 -01. 2. Data on file at Abbott. Report WC#90263783. Products are in development and are not yet available for sale. Any features or performance information presented are based on the current design goals for our next generation TAVI Technologies and may be subjected to revisions to meet the needs of the product development program. Caution: Investigational Device – limited by Federal Law (US) to investigational use. Not to be reproduced, distributed or excerpted. SJM-PTC-0519 -0169 | Item approved for OUS use only 16






PIs Raj Makkar and Michael Reardon

Jena. Valve Everdur™ Pericardial THV Design EYELETS INTERMEDIATE STRUTS THV ONION • PORCINE PERICARDIAL TISSUE • JASMINE (ANTI CALCIFICATION) TREATMENT THV CUSP • PORCINE PERICARDIAL TISSUE • (3) LEAFLETS SEWN TO FRAME NITINOL SUPPORT FRAME • SELF EXPANDING • LOW OVERALL HEIGHT • OPEN CELL DESIGN LOCATORS • TISSUE COVERED • RADIOPAQUE TANTALUM MARKER SEALING RING • (24) RHOMBI ELEMENTS

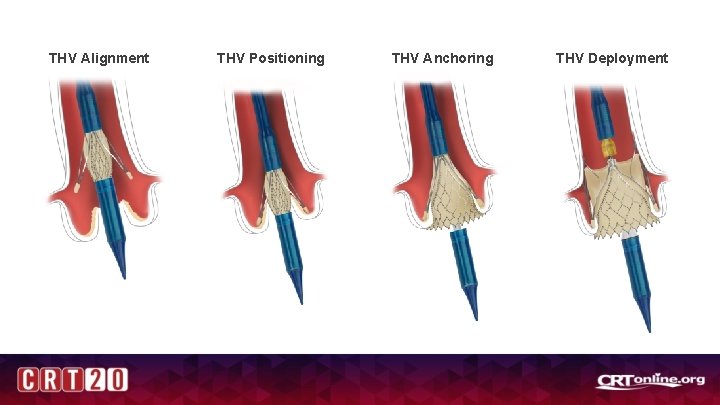
THV Alignment THV Positioning THV Anchoring THV Deployment

Study chair, M Leon PIs Torsten Vahl (IC) and Vinod Thourani (CS)

Jena. Valve AR Enrollment To Date 43 Patients Enrolled at 13 Centers Cedars Sinai Heart Institute, L. A. , CA Raj Makkar 9 Erasmus Medical Center, Rotterdam, NL Nicolas van Mieghem 2 Columbia University, New York, NY Susheel Kodali & Torsten Vahl 9 HDZ NRW, Bad Oeynhausen, Germany Tanja Rudolph and Sabine Bleiziffer 1 University of Cologne, Germany Stephan Baldus 6 Hamburg UKE, Hamburg, Germany Lenard Conradi 1 Washington Hospital Center, Washington D. C. Vinod Thourani 4 University of Halle, Germany Hendrik Treede 1 Berlin DHZ, Berlin, Germany Jörg Kempfert 4 University of Frankfurt, Germany Thomas Walther 1 Bonn University Hospital, Bonn, Germany Hendrik Treede 2 University of Washington, Seattle, WA Danny Dvir 1 Kerckhoff Heart Center, Bad Nauheim, Germany Won-Keun Kim 2

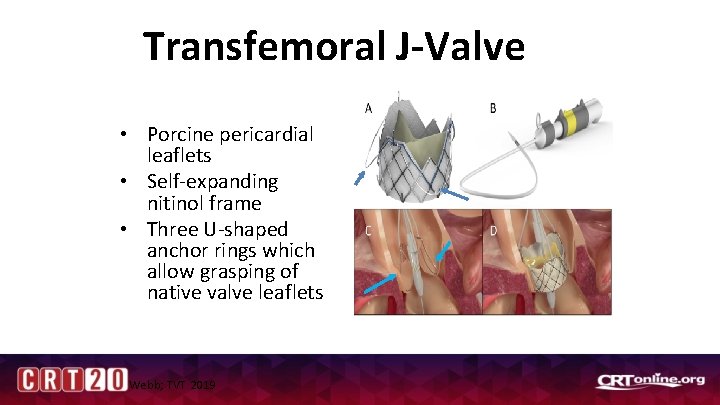
Transfemoral J-Valve • Porcine pericardial leaflets • Self-expanding nitinol frame • Three U-shaped anchor rings which allow grasping of native valve leaflets Webb; TVT 2019

Thank You