NEW MEXICO AMP ALLIANCE FOR MINORITY PARTICIPATION Source







- Slides: 15

NEW MEXICO AMP ALLIANCE FOR MINORITY PARTICIPATION Source tracking and community profiles of antibiotic resistant bacteria in wastewater samples Jesús Sigala and Felly Rose Montelya Mentor: Dr. Adrian Unc Plant and Environmental Sciences, NMSU

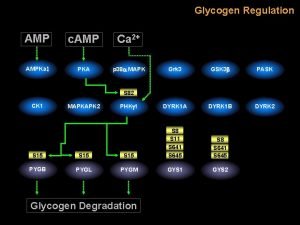
Research background and relevance Wastewater treatment plants treat waste generated from various sources including residential, industrial, schools, hospital and medical centers: screening and removal of non-degradable solids, physical removal of suspended solids to produce biosolids, biological treatment chemical treatment (chlorination) Treated wastewater is ultimately discharged to surface waters However, some microbes survive treatment; these may include pathogens and antibiotic resistant microbes Discharge of antibiotic resistant microbes affect water quality, has ecological concerns, and poses public health concerns

Wastewater samples Pretreatment—influent After treatment—effluent

Previous results Two observations: 1) For all antibiotics, the proportion of antibiotic resistant E. coli generally increases throughout the treatment with significant selection during aeration stage 2) Chlorination is most effective at eliminating resistant E. coli (but not perfect)

Research goals Assess a source tracking method that allows identification of pre-treatment sources Profile the antibiotic resistant bacterial community in wastewater using polymerase chain reaction-denaturing gradient gel electrophoresis (PCR-DGGE) Determine the impact of wastewater sources on the microbial quality of effluent discharge

Microbial Source Tracking Common method to identify source of pollution in food and water contamination sources Compare source and environmental samples Specific characteristics to compare include antibiotic resistance, mutation, or DNA fingerprint (Pillai and Vega, 2007)

Wastewater sampling Four lifting stations representing distinct sources Residential Industrial University Hospital Six stages of treatment Influent Primary clarifier Trickling (roughing) filters Aeration basin Secondary clarifier Chlorination tank Three samples from each location over a period of two days for composite samples

Lifting stations and wastewater treatment plant University wastewater lifting station Primary clarifiers at the treatment plant

Lab methods Selection of antibiotic resistant populations by plating on MH agar using antibiotic agar dilution method at four concentrations according to EUCAST (www. eucast. org) Antibiotics included: Erythromycin, Doxycycline, Cefaclor, Ciprofloxacin Extraction of DNA from the antibiotic selected populations (Mo. Bio extraction kit) Quantification of DNA by UV absorbance at 260 nm PCR amplification rpo. B primers (rpo. B 1698 F, with GC clamp at 5’ end, and rpo. B 2041 R) Thermocycler program as described by Peixoto et al. (2002) Polyacrylamide gels (6%) cast for DGGE (40% to 60% denaturing gradient) DGGE performed for 14 hours at 85 V DGGE gels were silver stained

Results and discussion Microbial growth from all samples and antibiotic concentrations DGGE and analysis for all samples to be completed DGGE will allow comparison of population diversity between different samples with interest in Dissimilarity between lifting stations Similarity between lifting stations and wastewater treatment samples

DGGE • • Lane 1: DGGE standard Lanes 2 -7: samples Lane 8: (empty) Lane 9: DGGE standard

Future goals Complete DGGE for all samples Analyze DGGE fingerprints using statistical methods Propose a novel source tracking method for identification of sources of antibiotic resistant microbial contaminants Propose future research aimed at improved targeting of the actual treatment protocols

Conclusion Sampling of lifting stations and wastewater treatment plant allows us to separate different sources PCR/DGGE can be used to profile bacterial diversity and develop a source tracking method Source impact on antibiotic resistance microbial load and community profile in wastewater treatment can be determined

Acknowledgements NM AMP NSF Grant #NSF HRD 083171 and NM WRRI for their support and interest in our research Dr. Unc for the support, guidance, review, and assistance during sampling and lab work Jeanne Garland, Polina Chemishanova, Gloria Vasquez, Joy Pugh, and other AMP staff who contributed in various ways to the research project

References Bitton, G. 2005. Wastewater microbiology, 3 rd ed. Wiley, Hoboken, New Jersey. Dahllöf, I. , H. Baillie, and S. Kjelleberg. 2000. rpo. B-Based microbial community analysis avoids limitations inherent in 16 S r. RNA gene intraspecies heterogeneity. Applied and Environmental Microbiology. 66: 3376 -3380. Felske, A. , and A. M. Osborn. 2005. DNA fingerprinting of microbial communities. In A. M. Osborn and C. J. Smith (ed. ), Molecular microbial ecology. Taylor & Francis Group, New York. Peixoto, R. S. , H. L. da Costa Countinho, N. G. Rumjaneck, A. Macrae, and A. S. Rosado. 2002. Use of rpo. B and 16 S r. RNA genes to analyse bacterial diversity of a tropical soil using PCR and DGGE. Letters in Applied Microbiology. 35: 316 -320. Pillai, S. , and E. Vega. 2007. Molecular detection and characterization tools. In J. W. Santo Domingo and M. J. Sadowsky (ed. ) Microbial source tracking. ASM, Washington. Wiegand, I. , K. Hilpert, and R. E. W. Hancock. 2008. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nature Protocols. 3: 163 -175.
 New mexico amp
New mexico amp Total participation constraint adalah
Total participation constraint adalah Op amp ideal and practical
Op amp ideal and practical Source follower op amp
Source follower op amp New nicotine alliance
New nicotine alliance Research alliance for nyc schools
Research alliance for nyc schools Timeline of new mexico history
Timeline of new mexico history New mexico medical insurance pool
New mexico medical insurance pool Interlock license new mexico
Interlock license new mexico New mexico brain injury resource center
New mexico brain injury resource center Acd 31102
Acd 31102 Nm pmp aware
Nm pmp aware New mexico ffa
New mexico ffa Cte school new mexico
Cte school new mexico Meteor crater new mexico
Meteor crater new mexico New mexico tech
New mexico tech