Nanoscience What Is It What Makes Nanoscience so



- Slides: 18

Nanoscience What Is It? What Makes Nanoscience so Different?

Nanoscience What units would be appropriate to measure the radii of atoms? Predict how many atoms you think a medium-sized nanoscale particle might contain. What Makes Nanoscience so Different? © Mc. REL 2009 2

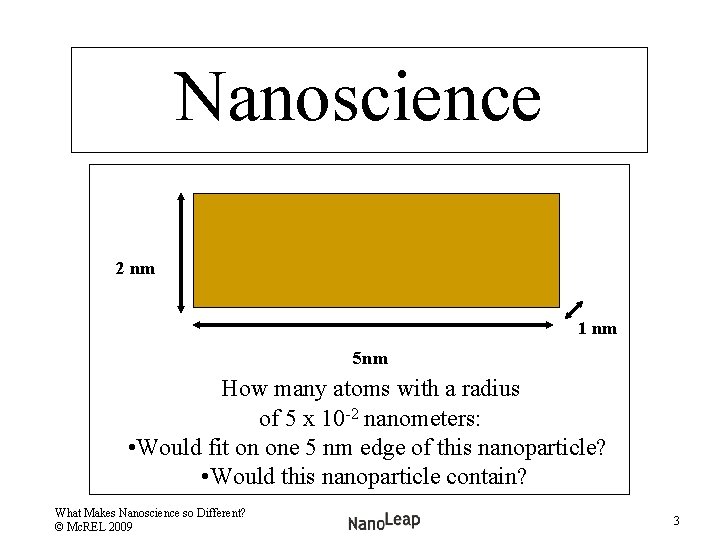
Nanoscience 2 nm 1 nm 5 nm How many atoms with a radius of 5 x 10 -2 nanometers: • Would fit on one 5 nm edge of this nanoparticle? • Would this nanoparticle contain? What Makes Nanoscience so Different? © Mc. REL 2009 3

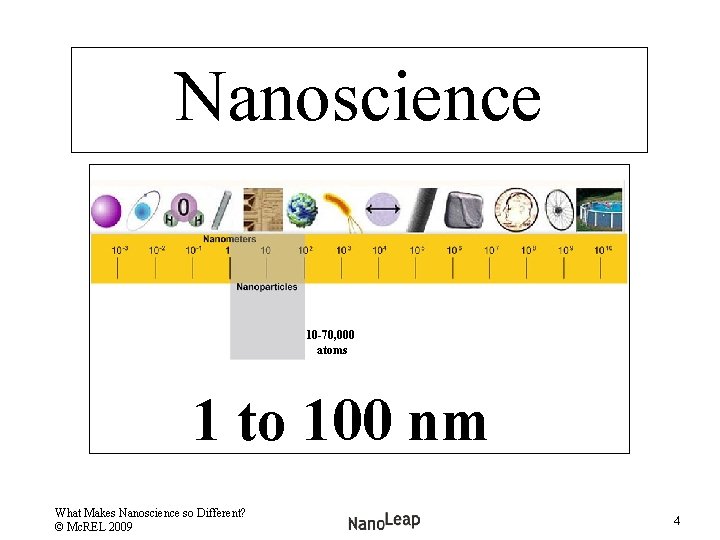
Nanoscience 10 -70, 000 atoms 1 to 100 nm What Makes Nanoscience so Different? © Mc. REL 2009 4

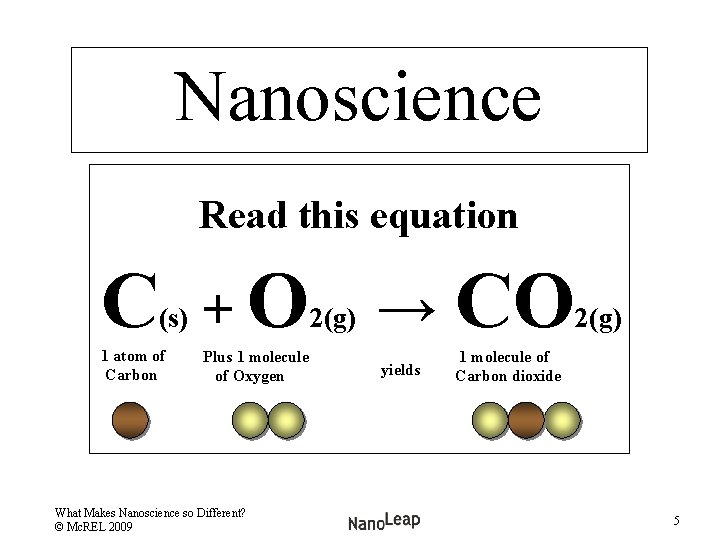
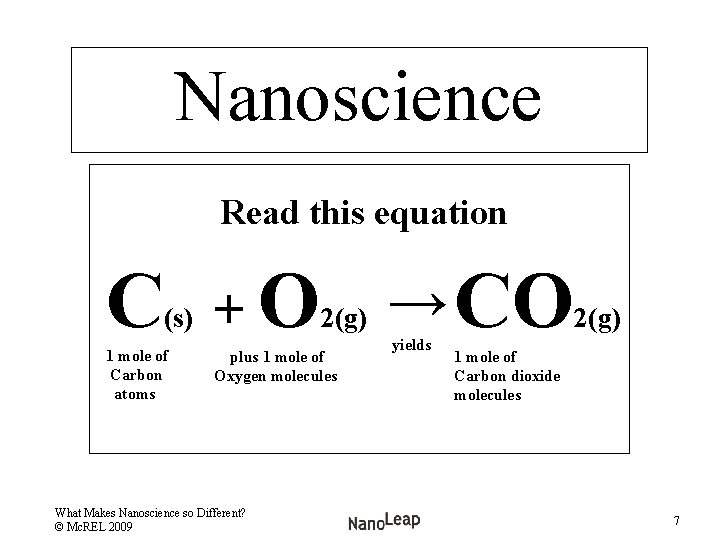
Nanoscience Read this equation C (s) 1 atom of Carbon + O 2(g) → CO Plus 1 molecule of Oxygen 1 molecule of Carbon dioxide What Makes Nanoscience so Different? © Mc. REL 2009 yields 2(g) 5

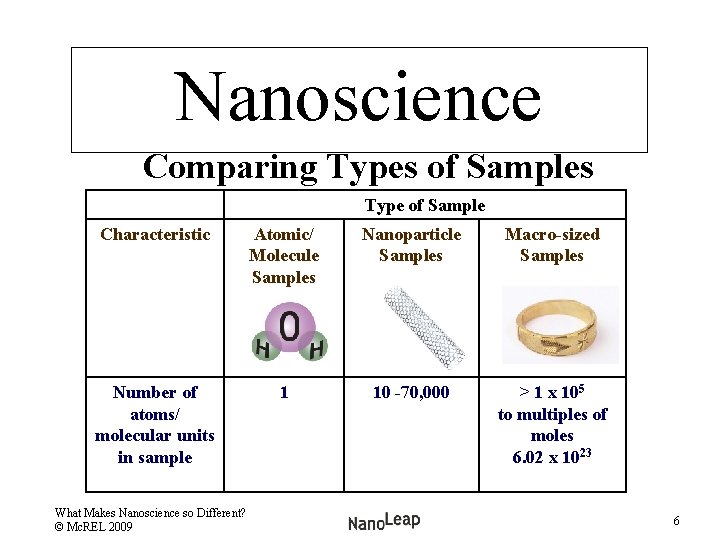
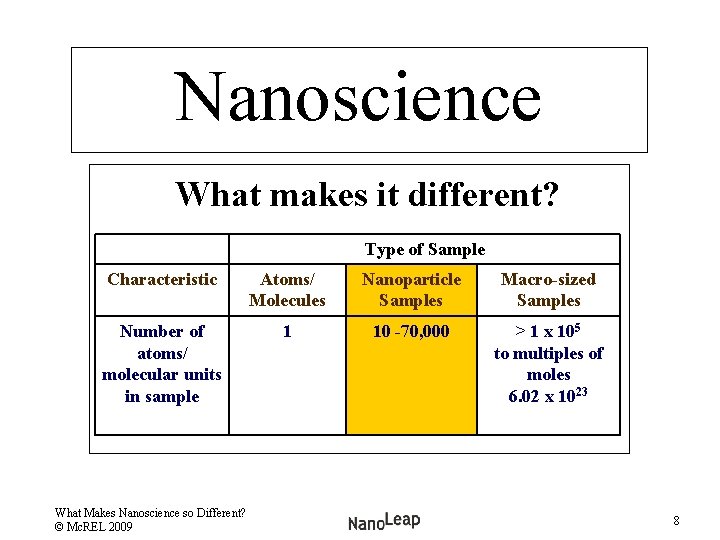
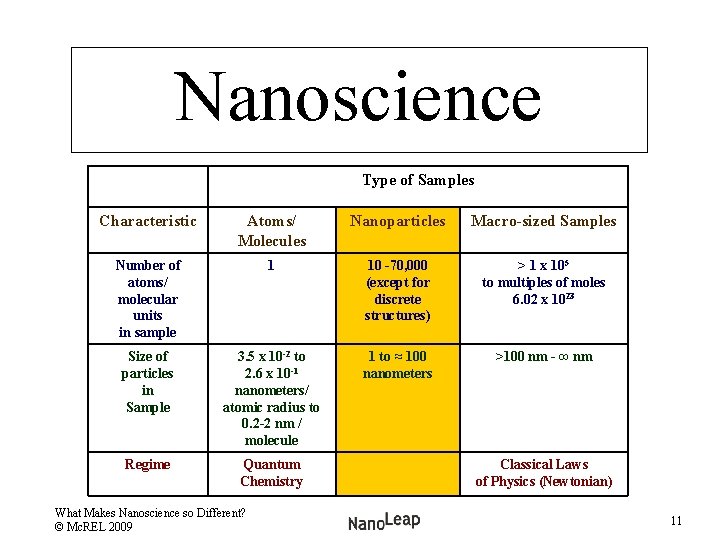
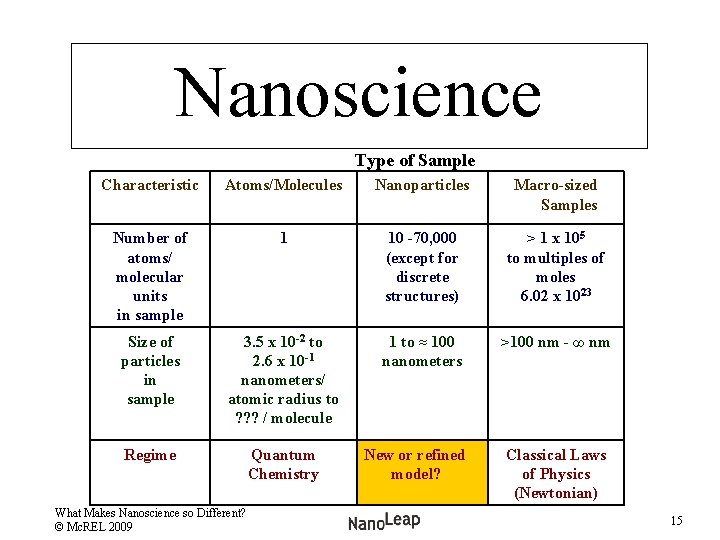
Nanoscience Comparing Types of Samples Type of Sample Characteristic Atomic/ Molecule Samples Nanoparticle Samples Macro-sized Samples Number of atoms/ molecular units in sample 1 10 -70, 000 > 1 x 105 to multiples of moles 6. 02 x 1023 What Makes Nanoscience so Different? © Mc. REL 2009 6

Nanoscience Read this equation C (s) 1 mole of Carbon atoms + O 2(g) → CO 2(g) plus 1 mole of Oxygen molecules What Makes Nanoscience so Different? © Mc. REL 2009 yields 1 mole of Carbon dioxide molecules 7

Nanoscience What makes it different? Type of Sample Characteristic Atoms/ Molecules Nanoparticle Samples Macro-sized Samples Number of atoms/ molecular units in sample 1 10 -70, 000 > 1 x 105 to multiples of moles 6. 02 x 1023 What Makes Nanoscience so Different? © Mc. REL 2009 8

Nanoscience Did we work with any nanosized samples? Fewer than 70, 000 atoms per particle? What Makes Nanoscience so Different? © Mc. REL 2009 9

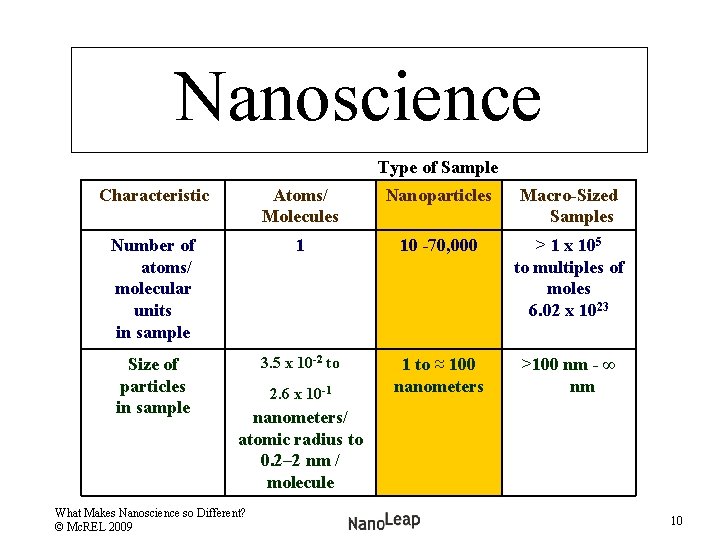
Nanoscience Type of Sample Characteristic Atoms/ Molecules Nanoparticles Macro-Sized Samples Number of atoms/ molecular units in sample 1 10 -70, 000 > 1 x 105 to multiples of moles 6. 02 x 1023 Size of particles in sample 3. 5 x 10 -2 to 1 to ≈ 100 nanometers >100 nm - ∞ nm 2. 6 x 10 -1 nanometers/ atomic radius to 0. 2– 2 nm / molecule What Makes Nanoscience so Different? © Mc. REL 2009 10

Nanoscience Type of Samples Characteristic Atoms/ Molecules Nanoparticles Macro-sized Samples Number of atoms/ molecular units in sample 1 10 -70, 000 (except for discrete structures) > 1 x 105 to multiples of moles 6. 02 x 1023 Size of particles in Sample 3. 5 x 10 -2 to 2. 6 x 10 -1 nanometers/ atomic radius to 0. 2 -2 nm / molecule 1 to ≈ 100 nanometers >100 nm - ∞ nm Regime Quantum Chemistry What Makes Nanoscience so Different? © Mc. REL 2009 Classical Laws of Physics (Newtonian) 11

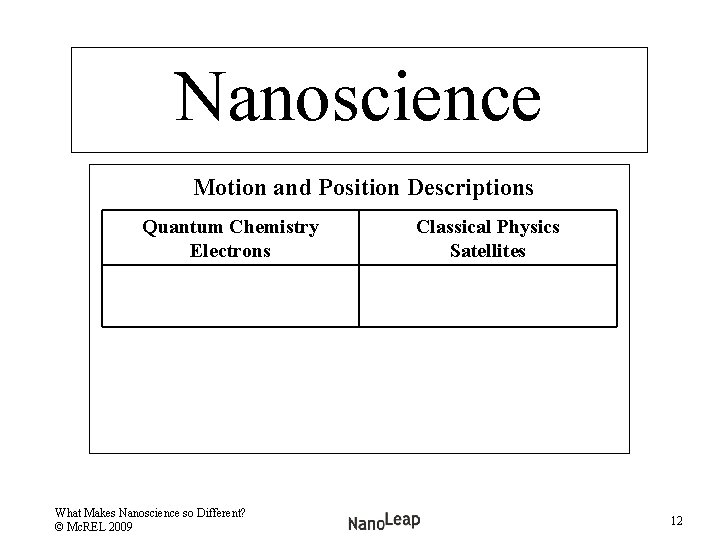
Nanoscience Motion and Position Descriptions Quantum Chemistry Electrons What Makes Nanoscience so Different? © Mc. REL 2009 Classical Physics Satellites 12

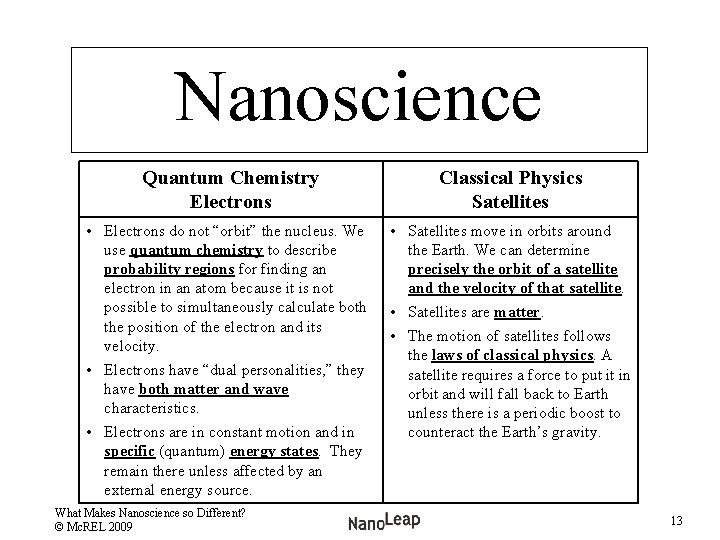
Nanoscience Quantum Chemistry Electrons Classical Physics Satellites • Electrons do not “orbit” the nucleus. We use quantum chemistry to describe probability regions for finding an electron in an atom because it is not possible to simultaneously calculate both the position of the electron and its velocity. • Electrons have “dual personalities, ” they have both matter and wave characteristics. • Electrons are in constant motion and in specific (quantum) energy states. They remain there unless affected by an external energy source. • Satellites move in orbits around the Earth. We can determine precisely the orbit of a satellite and the velocity of that satellite. • Satellites are matter. • The motion of satellites follows the laws of classical physics. A satellite requires a force to put it in orbit and will fall back to Earth unless there is a periodic boost to counteract the Earth’s gravity. What Makes Nanoscience so Different? © Mc. REL 2009 13

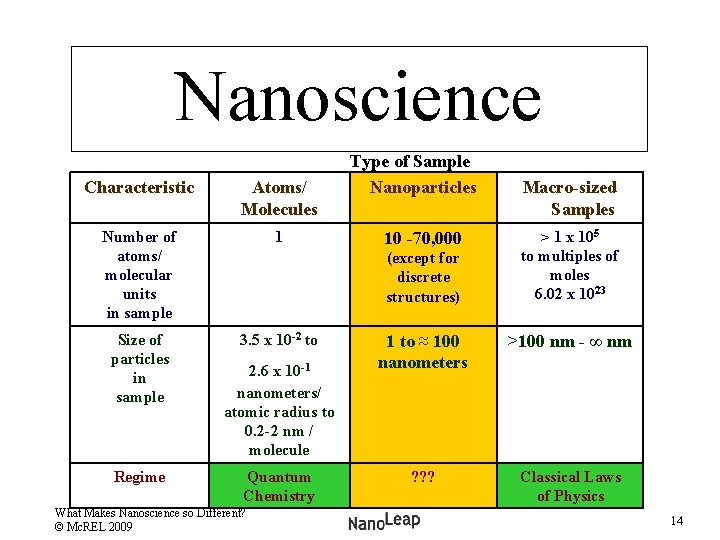
Nanoscience Characteristic Atoms/ Molecules Number of atoms/ molecular units in sample 1 Size of particles in sample 3. 5 x 10 -2 to Regime Type of Sample Nanoparticles 10 -70, 000 (except for discrete structures) 2. 6 x 10 -1 nanometers/ atomic radius to 0. 2 -2 nm / molecule Quantum Chemistry What Makes Nanoscience so Different? © Mc. REL 2009 Macro-sized Samples > 1 x 105 to multiples of moles 6. 02 x 1023 1 to ≈ 100 nanometers >100 nm - ∞ nm ? ? ? Classical Laws of Physics 14

Nanoscience Type of Sample Characteristic Atoms/Molecules Nanoparticles Macro-sized Samples Number of atoms/ molecular units in sample 1 10 -70, 000 (except for discrete structures) > 1 x 105 to multiples of moles 6. 02 x 1023 Size of particles in sample 3. 5 x 10 -2 to 2. 6 x 10 -1 nanometers/ atomic radius to ? ? ? / molecule 1 to ≈ 100 nanometers >100 nm - ∞ nm Regime Quantum Chemistry New or refined model? Classical Laws of Physics (Newtonian) What Makes Nanoscience so Different? © Mc. REL 2009 15

Nanoscience sometimes are not sure WHAT they are! What Makes Nanoscience so Different? © Mc. REL 2009 16

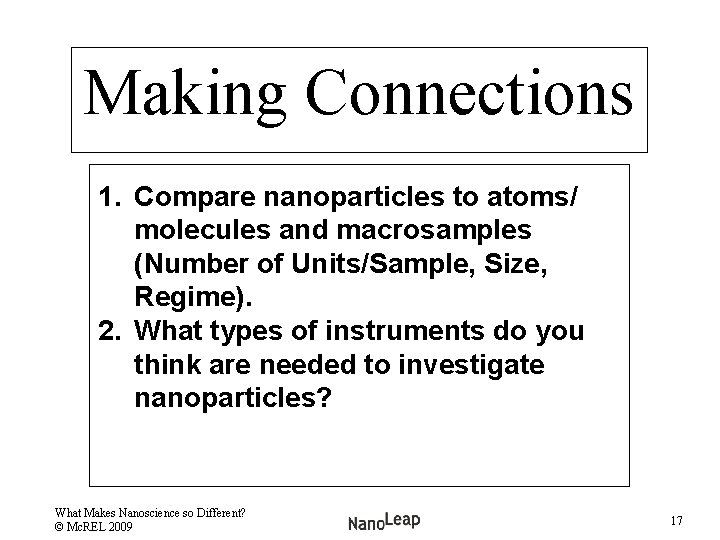
Making Connections 1. Compare nanoparticles to atoms/ molecules and macrosamples (Number of Units/Sample, Size, Regime). 2. What types of instruments do you think are needed to investigate nanoparticles? What Makes Nanoscience so Different? © Mc. REL 2009 17

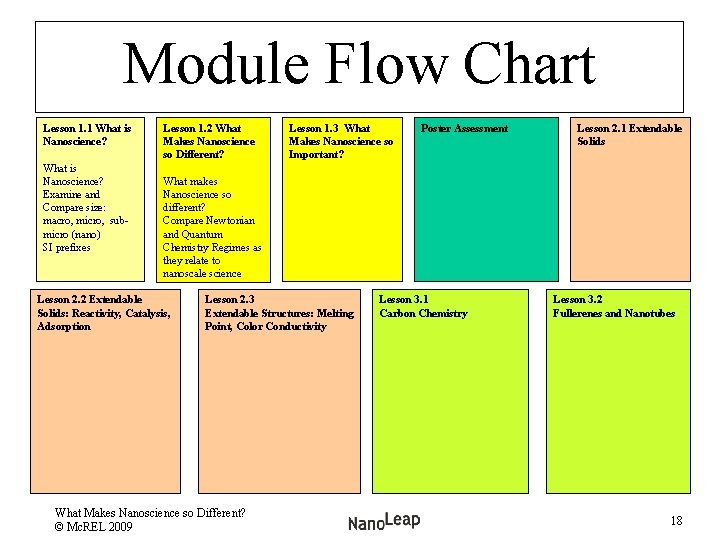
Module Flow Chart Lesson 1. 1 What is Nanoscience? Examine and Compare size: macro, micro, submicro (nano) SI prefixes Lesson 1. 2 What Makes Nanoscience so Different? Lesson 1. 3 What Makes Nanoscience so Important? Poster Assessment Lesson 2. 1 Extendable Solids What makes Nanoscience so different? Compare Newtonian and Quantum Chemistry Regimes as they relate to nanoscale science Lesson 2. 2 Extendable Solids: Reactivity, Catalysis, Adsorption Lesson 2. 3 Extendable Structures: Melting Point, Color Conductivity What Makes Nanoscience so Different? © Mc. REL 2009 Lesson 3. 1 Carbon Chemistry Lesson 3. 2 Fullerenes and Nanotubes 18