Nanoelectronics 11 Atsufumi Hirohata Department of Electronic Engineering


- Slides: 17

Nanoelectronics 11 Atsufumi Hirohata Department of Electronic Engineering 09: 00 (online) & 12: 00 (SLB 118 & online) Monday, 22/February/2021

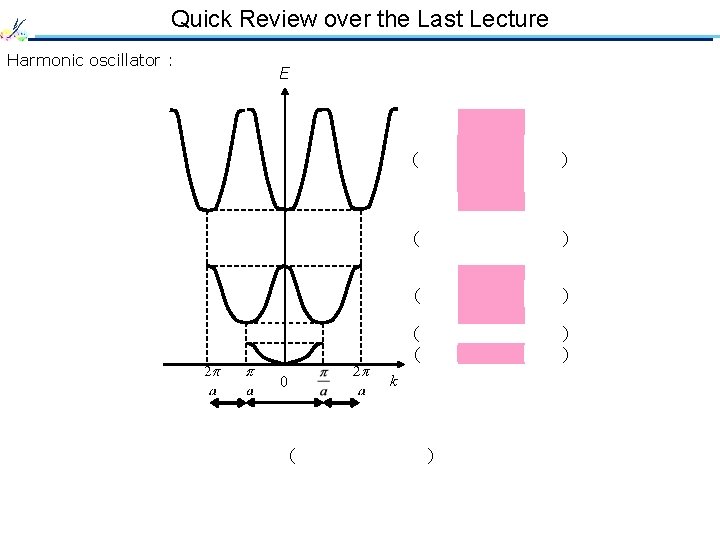
Quick Review over the Last Lecture Harmonic oscillator : E ( Allowed band ) ( Forbidden band ) ( Allowed band ) k 0 2 nd 1 st 2 nd ( Brillouin zone )

Contents of Nanoelectonics I. Introduction to Nanoelectronics (01) 01 Micro- or nano-electronics ? II. Electromagnetism (02 & 03) 02 Maxwell equations 03 Scholar and vector potentials III. Basics of quantum mechanics (04 ~ 06) 04 History of quantum mechanics 1 05 History of quantum mechanics 2 06 Schrödinger equation IV. Applications of quantum mechanics (07, 10, 11, 13 & 14) 07 Quantum well 10 Harmonic oscillator 11 Magnetic spin V. Nanodevices (08, 09, 12, 15 ~ 18) 08 Tunnelling nanodevices 09 Nanomeasurements

11 Magnetic spin Origin of magnetism • Spin / orbital moment • • • Paramagnetism • Ferromagnetism Antiferromagnetism

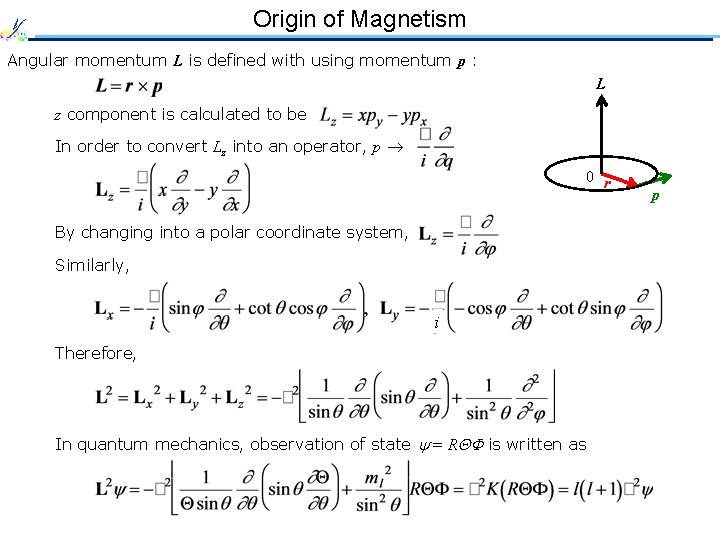
Origin of Magnetism Angular momentum L is defined with using momentum p : L z component is calculated to be In order to convert Lz into an operator, p 0 r By changing into a polar coordinate system, Similarly, i Therefore, In quantum mechanics, observation of state = R is written as p

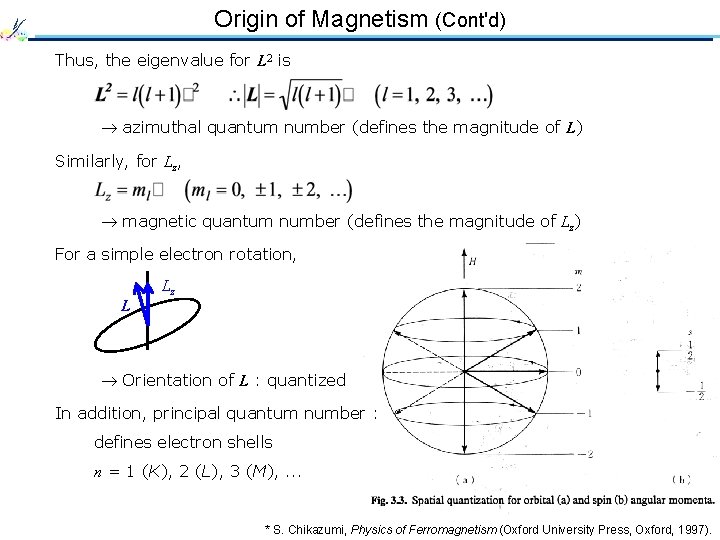
Origin of Magnetism (Cont'd) Thus, the eigenvalue for L 2 is azimuthal quantum number (defines the magnitude of L) Similarly, for Lz, magnetic quantum number (defines the magnitude of Lz) For a simple electron rotation, L Lz Orientation of L : quantized In addition, principal quantum number : defines electron shells n = 1 (K), 2 (L), 3 (M), . . . * S. Chikazumi, Physics of Ferromagnetism (Oxford University Press, Oxford, 1997).

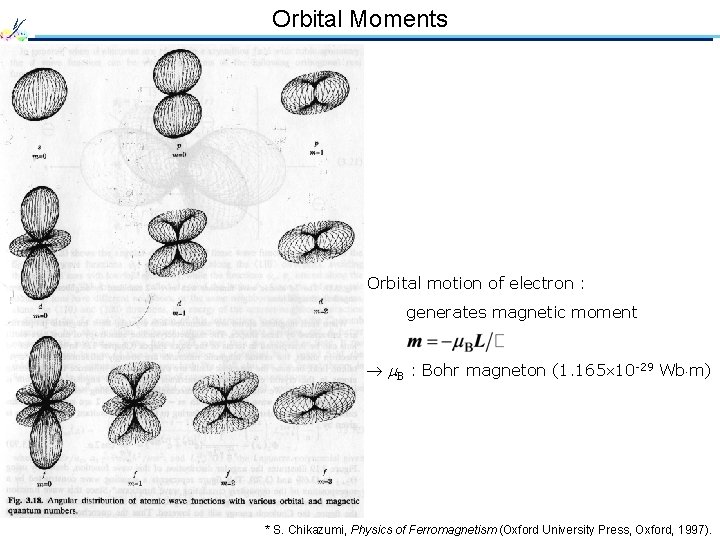
Orbital Moments Orbital motion of electron : generates magnetic moment B : Bohr magneton (1. 165 10 -29 Wb m) * S. Chikazumi, Physics of Ferromagnetism (Oxford University Press, Oxford, 1997).

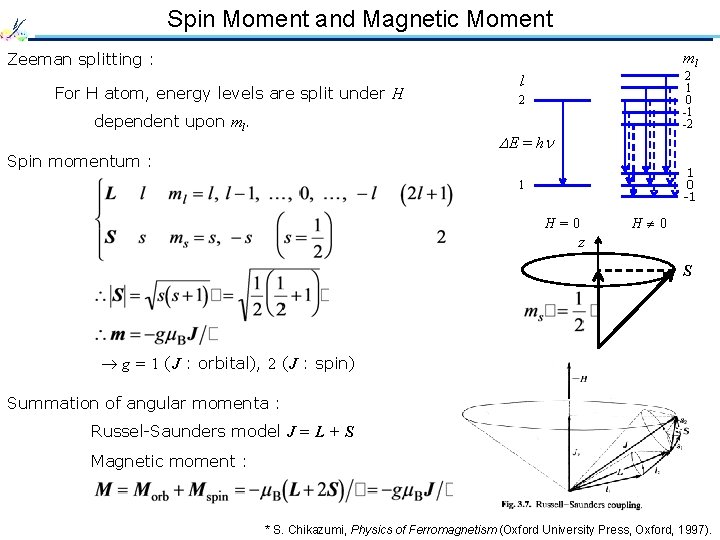
Spin Moment and Magnetic Moment ml Zeeman splitting : For H atom, energy levels are split under H dependent upon ml. 2 1 0 -1 -2 l 2 E = h Spin momentum : 1 0 -1 1 H=0 H 0 z S g = 1 (J : orbital), 2 (J : spin) Summation of angular momenta : Russel-Saunders model J = L + S Magnetic moment : * S. Chikazumi, Physics of Ferromagnetism (Oxford University Press, Oxford, 1997).

Magnetic Moment

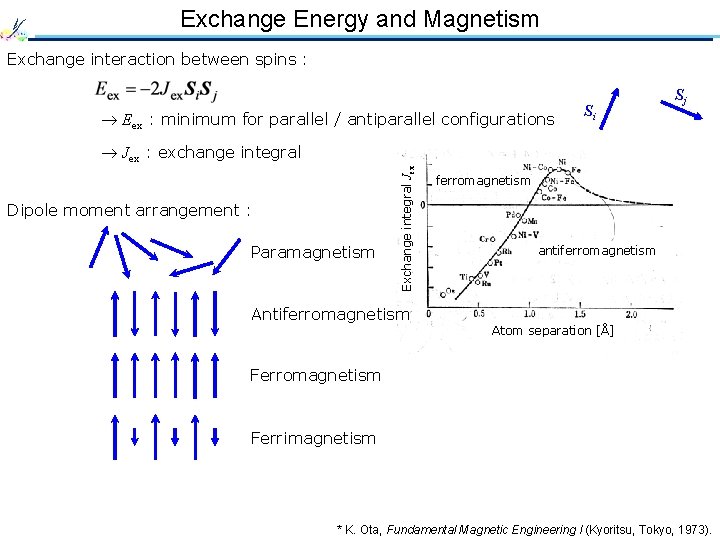
Exchange Energy and Magnetism Exchange interaction between spins : Eex : minimum for parallel / antiparallel configurations Si Sj Dipole moment arrangement : Paramagnetism Exchange integral Jex : exchange integral Antiferromagnetism antiferromagnetism Atom separation [Å] Ferromagnetism Ferrimagnetism * K. Ota, Fundamental Magnetic Engineering I (Kyoritsu, Tokyo, 1973).

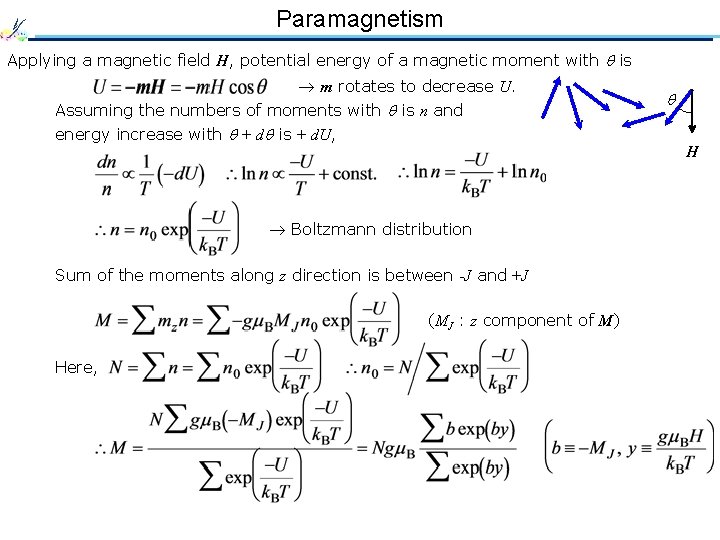
Paramagnetism Applying a magnetic field H, potential energy of a magnetic moment with is m rotates to decrease U. Assuming the numbers of moments with is n and energy increase with + d is + d. U, Boltzmann distribution Sum of the moments along z direction is between -J and +J (MJ : z component of M) Here, H

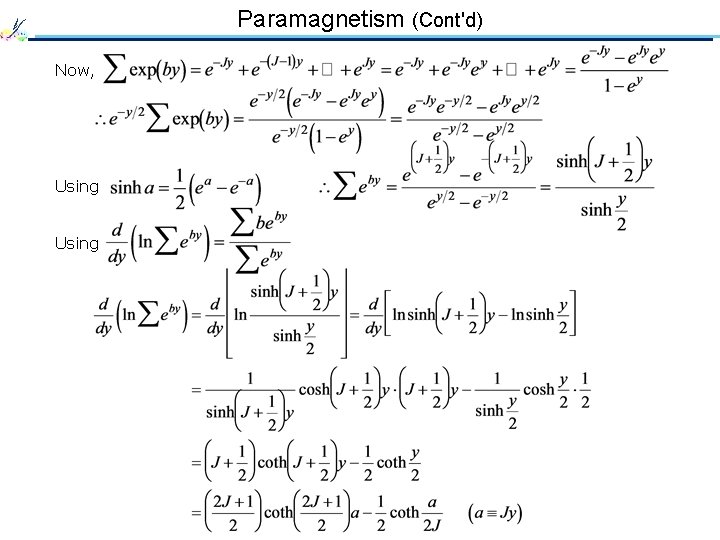
Paramagnetism (Cont'd) Now, Using

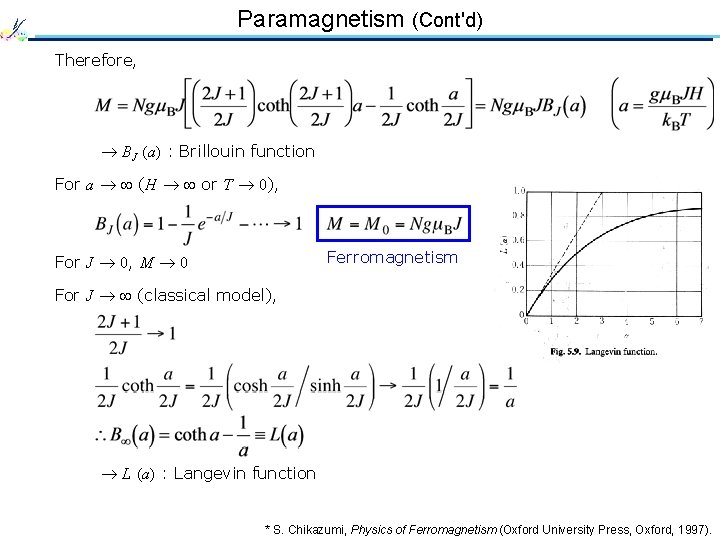
Paramagnetism (Cont'd) Therefore, BJ (a) : Brillouin function For a (H or T 0), Ferromagnetism For J 0, M 0 For J (classical model), L (a) : Langevin function * S. Chikazumi, Physics of Ferromagnetism (Oxford University Press, Oxford, 1997).

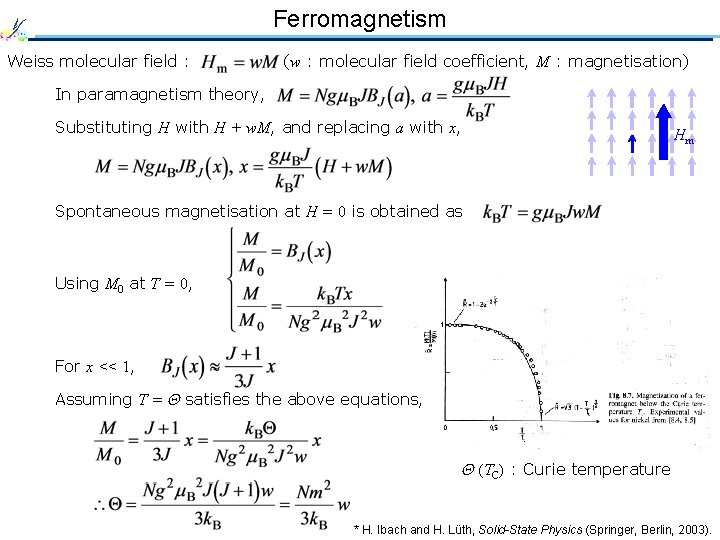
Ferromagnetism Weiss molecular field : (w : molecular field coefficient, M : magnetisation) In paramagnetism theory, Substituting H with H + w. M, and replacing a with x, Hm Spontaneous magnetisation at H = 0 is obtained as Using M 0 at T = 0, For x << 1, Assuming T = satisfies the above equations, (TC) : Curie temperature * H. Ibach and H. Lüth, Solid-State Physics (Springer, Berlin, 2003).

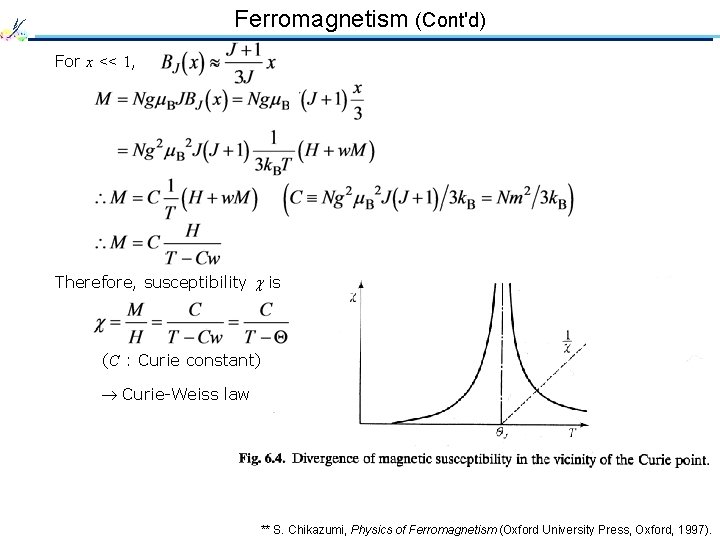
Ferromagnetism (Cont'd) For x << 1, Therefore, susceptibility is (C : Curie constant) Curie-Weiss law ** S. Chikazumi, Physics of Ferromagnetism (Oxford University Press, Oxford, 1997).

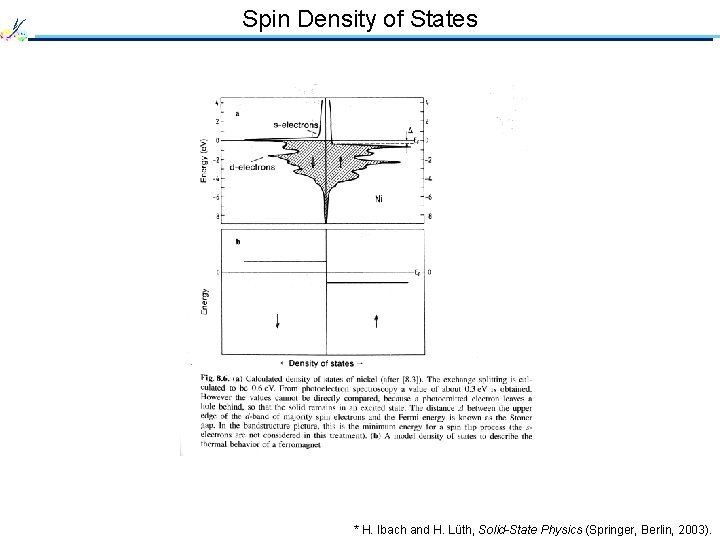
Spin Density of States * H. Ibach and H. Lüth, Solid-State Physics (Springer, Berlin, 2003).

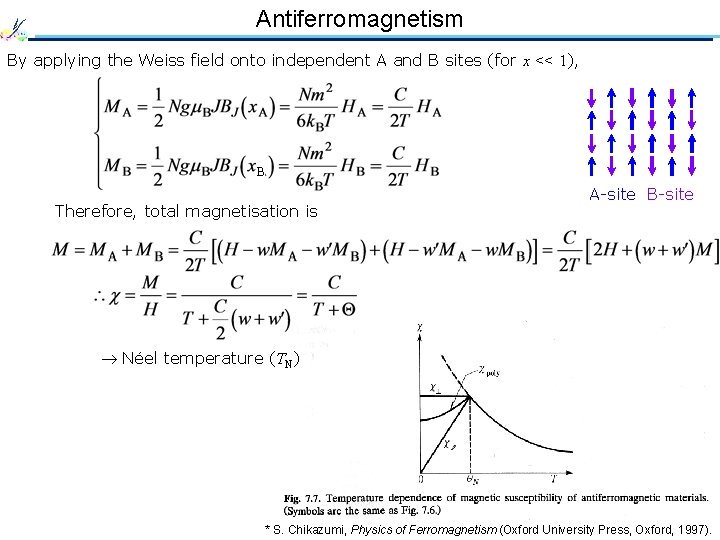
Antiferromagnetism By applying the Weiss field onto independent A and B sites (for x << 1), B Therefore, total magnetisation is A-site B-site Néel temperature (TN) * S. Chikazumi, Physics of Ferromagnetism (Oxford University Press, Oxford, 1997).
 Atsufumi hirohata
Atsufumi hirohata Atsufumi hirohata
Atsufumi hirohata Atsufumi hirohata
Atsufumi hirohata Atsufumi hirohata
Atsufumi hirohata Atsufumi hirohata
Atsufumi hirohata Atsufumi hirohata
Atsufumi hirohata Atsufumi hirohata
Atsufumi hirohata Nanoelectronics
Nanoelectronics Semiconductor examples
Semiconductor examples Nanoelectronics
Nanoelectronics An electronic is the electronic exchange of money or scrip
An electronic is the electronic exchange of money or scrip Electronic field production
Electronic field production Electronic engineering
Electronic engineering Electronic engineering
Electronic engineering Electrical design automation
Electrical design automation Electronic engineering
Electronic engineering Electronic engineering adalah
Electronic engineering adalah Electronic engineering adalah
Electronic engineering adalah





























