Molarity M M moles of solute Liters of






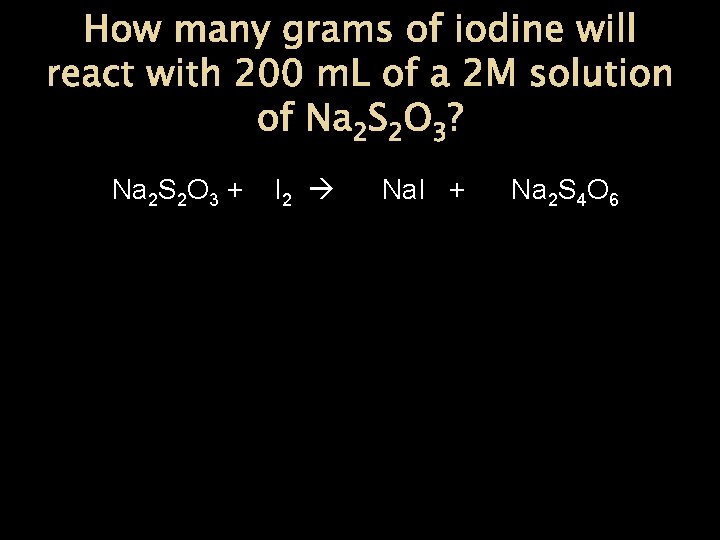
- Slides: 9

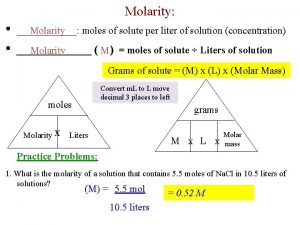
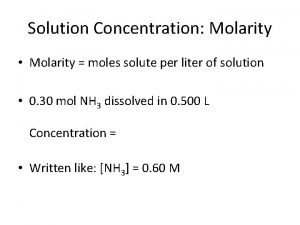
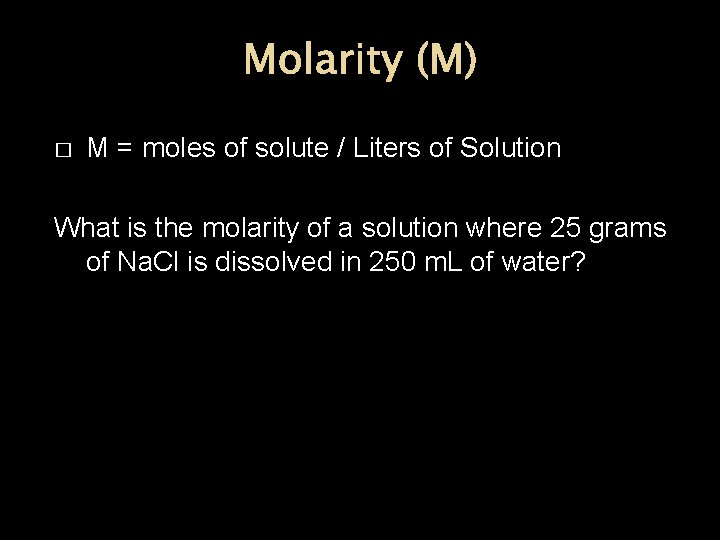
Molarity (M) � M = moles of solute / Liters of Solution What is the molarity of a solution where 25 grams of Na. Cl is dissolved in 250 m. L of water?

Dilutions � Most chemicals come from the chemical company in the most concentrated form. � For example, when we order hydrochloric acid it comes in a big stock bottle with a concentration of 15 M. � Most of the labs that we do only require 1. 0 M HCl. � How do we make a 1. 0 M solution?

Dilution Problems � A stock solution of 1. 00 M Na. Cl is available. How many milliliters are needed to make 100. 0 m. L of 0. 750 M Na. Cl? M 1 V 1 = M 2 V 2

Practice—How Many Grams of Cu. SO 4 5 H 2 O (MM 249. 69) are in 250. 0 m. L of a 1. 00 M Solution? Tro's Introductory Chemistry, Chapter 13 4

Practice—How Would You Prepare 100. 0 m. L of 0. 100 M K 2 SO 4 (MM = 174. 26)? Tro's Introductory Chemistry, Chapter 13 5

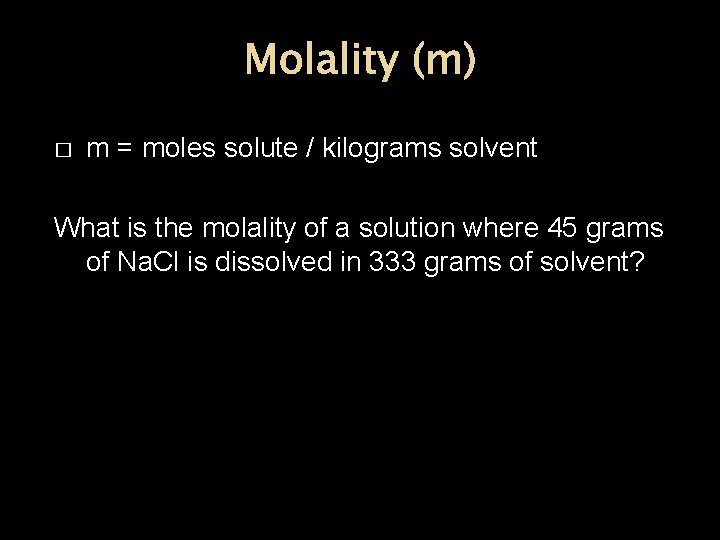
Molality (m) � m = moles solute / kilograms solvent What is the molality of a solution where 45 grams of Na. Cl is dissolved in 333 grams of solvent?

How Many Liters of 0. 115 M KI Is Needed to React with 0. 104 L of a 0. 225 M Pb(NO 3)2? 2 KI(aq) + Pb(NO 3)2(aq) 2 KNO 3(aq) + Pb. I 2(s) Tro's Introductory Chemistry, Chapter 13 7

How Many Liters of 0. 0623 M Ba(OH)2(aq) Are Needed to React with 0. 438 L of 0. 107 M HCl? Ba(OH)2(aq) + 2 HCl(aq) Ba. Cl 2(aq) + 2 H 2 O(l) Tro's Introductory Chemistry, Chapter 13 8
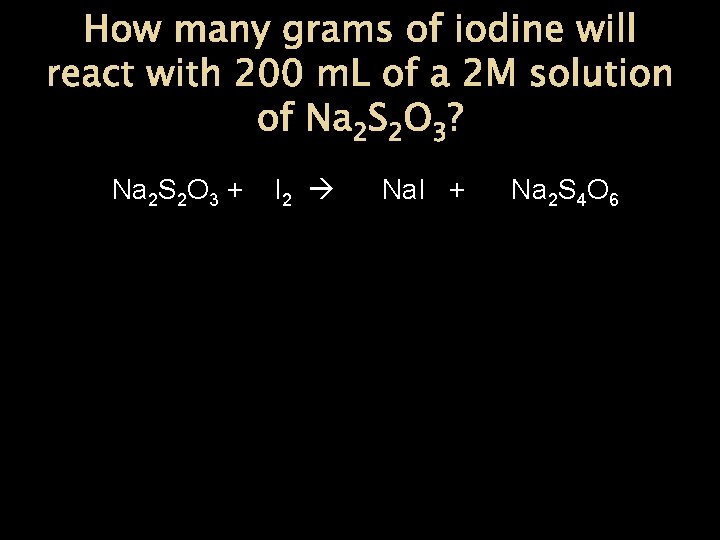
How many grams of iodine will react with 200 m. L of a 2 M solution of Na 2 S 2 O 3? Na 2 S 2 O 3 + I 2 Na. I + Na 2 S 4 O 6
 Mass/molar mass
Mass/molar mass Molarity is the number of moles of solute dissolved in
Molarity is the number of moles of solute dissolved in Liters to moles to grams
Liters to moles to grams What operation yields the number of moles of solute
What operation yields the number of moles of solute Molarity formula triangle
Molarity formula triangle How to find moles from molarity
How to find moles from molarity Solution stoichiometry
Solution stoichiometry If i place 3 moles of n2 and 4 moles of o2 in a 35l
If i place 3 moles of n2 and 4 moles of o2 in a 35l Oxygen liters per minute chart
Oxygen liters per minute chart Convert 12 liters to barrels
Convert 12 liters to barrels