Molarity Molarity moles of solute per liter of



- Slides: 6

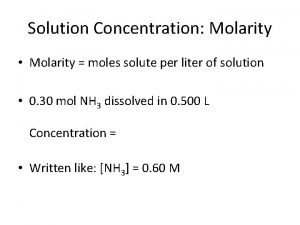
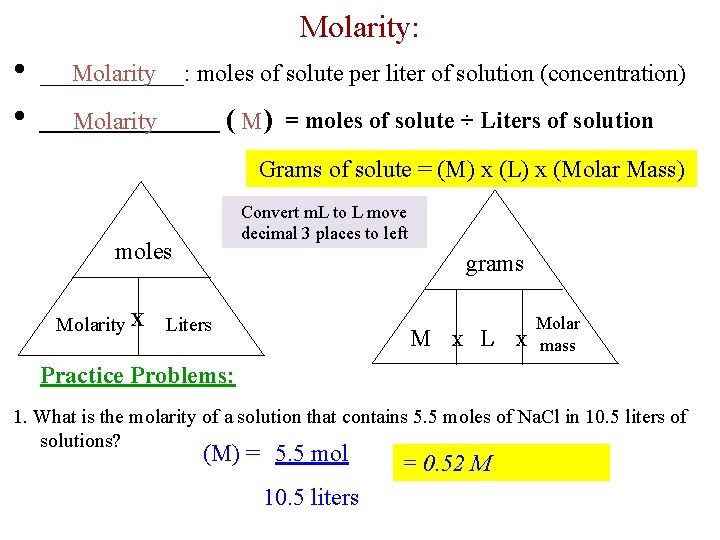
Molarity: • • Molarity ______: moles of solute per liter of solution (concentration) ________ ( M ) = moles of solute ÷ Liters of solution Molarity Grams of solute = (M) x (L) x (Molar Mass) Convert m. L to L move decimal 3 places to left moles Molarity X grams Liters M X L Molar X mass Practice Problems: 1. What is the molarity of a solution that contains 5. 5 moles of Na. Cl in 10. 5 liters of solutions? (M) = 5. 5 mol 10. 5 liters = 0. 52 M

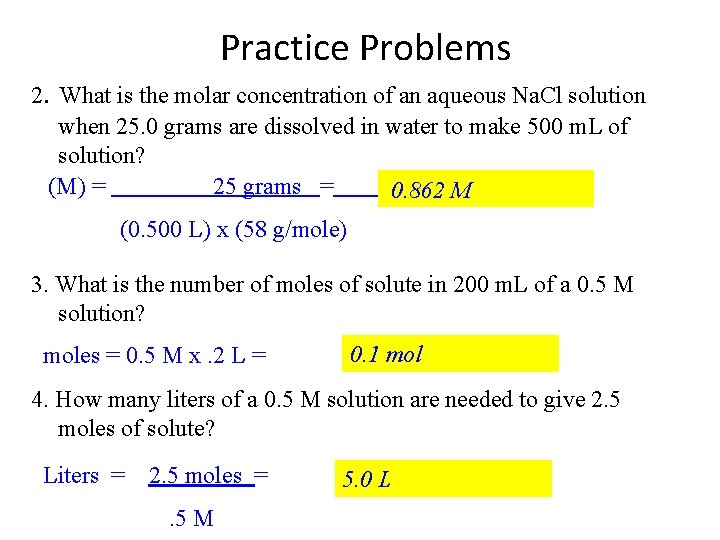
Practice Problems 2. What is the molar concentration of an aqueous Na. Cl solution when 25. 0 grams are dissolved in water to make 500 m. L of solution? (M) = 25 grams = 0. 862 M (0. 500 L) x (58 g/mole) 3. What is the number of moles of solute in 200 m. L of a 0. 5 M solution? moles = 0. 5 M x. 2 L = 0. 1 mol 4. How many liters of a 0. 5 M solution are needed to give 2. 5 moles of solute? Liters = 2. 5 moles =. 5 M 5. 0 L

Making a Solution of a Required Concentration # of moles ÷ # of liters = Molarity


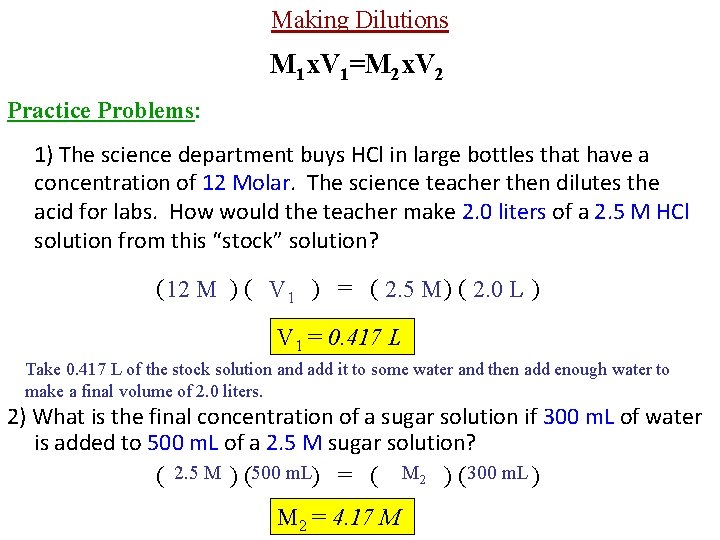
Making Dilutions • less concentrated by ____ adding Dilution: Making a solution _______ more ______. solvent Important: When diluting acids: “Add acid to water, do as you ought-er. ” A in W M 1 x. V 1=M 2 x. V 2 M 1 -- the initial concentration of the solution. V 1 -- the initial volume of the original solution that is going to be diluted with water. M 2 -- the final concentration of the solution after it’s diluted with water. V 2 -- the total volume of the final solution after it has been diluted with water.

Making Dilutions M 1 x. V 1=M 2 x. V 2 Practice Problems: 1) The science department buys HCl in large bottles that have a concentration of 12 Molar. The science teacher then dilutes the acid for labs. How would the teacher make 2. 0 liters of a 2. 5 M HCl solution from this “stock” solution? ( 12 M ) ( V 1 ) = ( 2. 5 M ) ( 2. 0 L ) V 1 = 0. 417 L Take 0. 417 L of the stock solution and add it to some water and then add enough water to make a final volume of 2. 0 liters. 2) What is the final concentration of a sugar solution if 300 m. L of water is added to 500 m. L of a 2. 5 M sugar solution? ( 2. 5 M ) (500 m. L) = ( M 2 ) ( 300 m. L ) M 2 = 4. 17 M
 Effect of temperature on solubility
Effect of temperature on solubility 27 miles per gallon into kilometers per liter
27 miles per gallon into kilometers per liter What operation yields the number of moles of solute
What operation yields the number of moles of solute Ada 2 gelas kosong berukuran 5 liter dan 3 liter
Ada 2 gelas kosong berukuran 5 liter dan 3 liter Ada 2 gelas kosong berukuran 5 liter dan 3 liter
Ada 2 gelas kosong berukuran 5 liter dan 3 liter Ada 2 gelas kosong berukuran 5 liter dan 3 liter
Ada 2 gelas kosong berukuran 5 liter dan 3 liter Ada 2 gelas kosong berukuran 5 liter dan 3 liter
Ada 2 gelas kosong berukuran 5 liter dan 3 liter