Minia University Faculty of Engineering Chemical Engineering Department



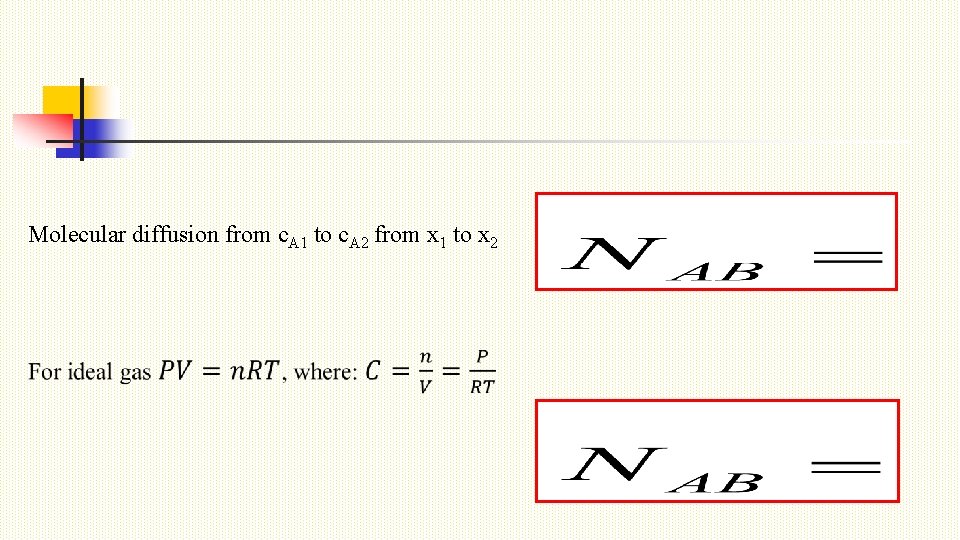

- Slides: 20

Minia University Faculty of Engineering Chemical Engineering Department Course Title: Mass Transfer Course Code: CHE 312 Third Year Course Coordinator: P r o f. Dr. Mohammad Shawky Week No. 2 CHAPTER TWO: PRINCIPLES of MASS TRANSFER

Contents n Diffusion phenomena n Molecular diffusion n Fick’s First Law of Diffusion (Steady state Law) n Fick’s Second Law of Diffusion ( Unsteady-state Law)

Diffusion phenomena n n n Fick’s law: linear relation between the rate of diffusion of chemical species and the concentration gradient of that species. Thermal diffusion: Diffusion due to a temperature gradient. Usually negligible unless the temperature gradient is very large. Pressure diffusion: Diffusion due to a pressure gradient. Usually negligible unless the pressure gradient is very large. Forced diffusion: Diffusion due to external force field acting on a molecule. Forced diffusion occurs when an electrical field is imposed on an electrolyte ( for example, in charging an automobile battery) Knudsen diffusion: Diffusion phenomena occur in porous solids.

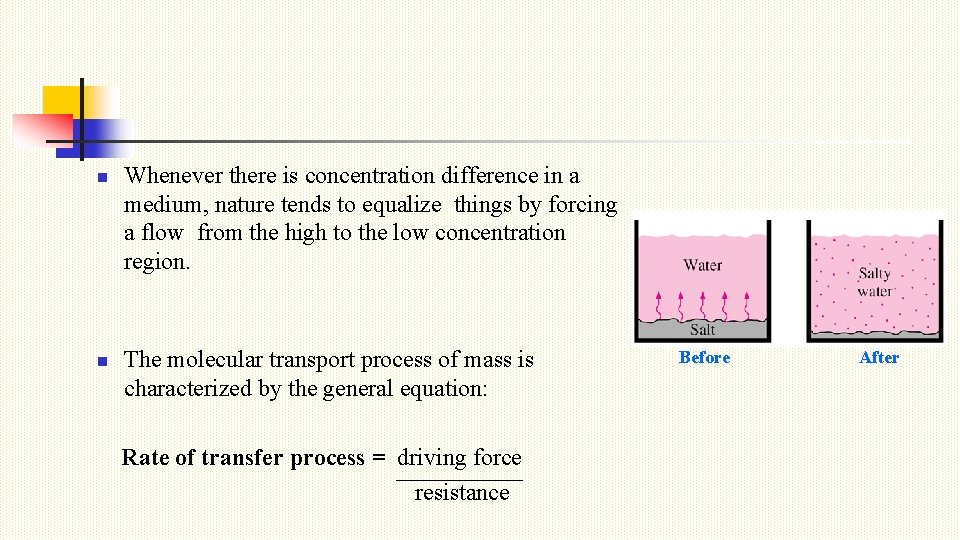
n n Whenever there is concentration difference in a medium, nature tends to equalize things by forcing a flow from the high to the low concentration region. The molecular transport process of mass is characterized by the general equation: Rate of transfer process = driving force resistance Before After

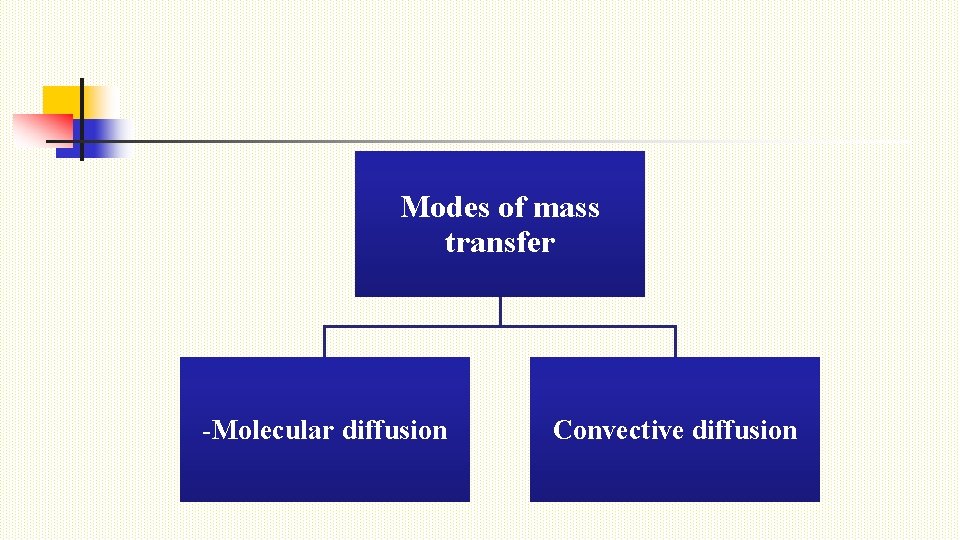
Modes of mass transfer -Molecular diffusion Convective diffusion

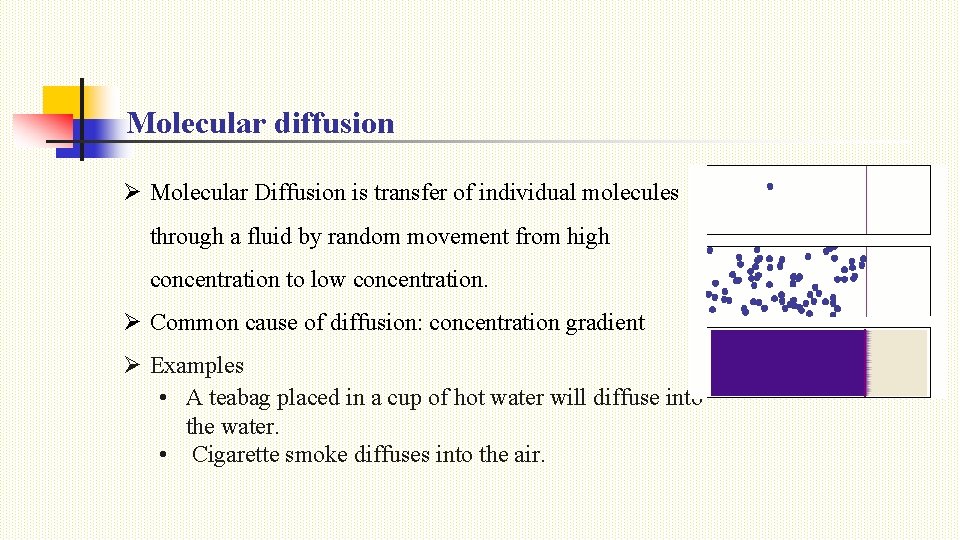
Molecular diffusion Ø Molecular Diffusion is transfer of individual molecules through a fluid by random movement from high concentration to low concentration. Ø Common cause of diffusion: concentration gradient Ø Examples • A teabag placed in a cup of hot water will diffuse into the water. • Cigarette smoke diffuses into the air.

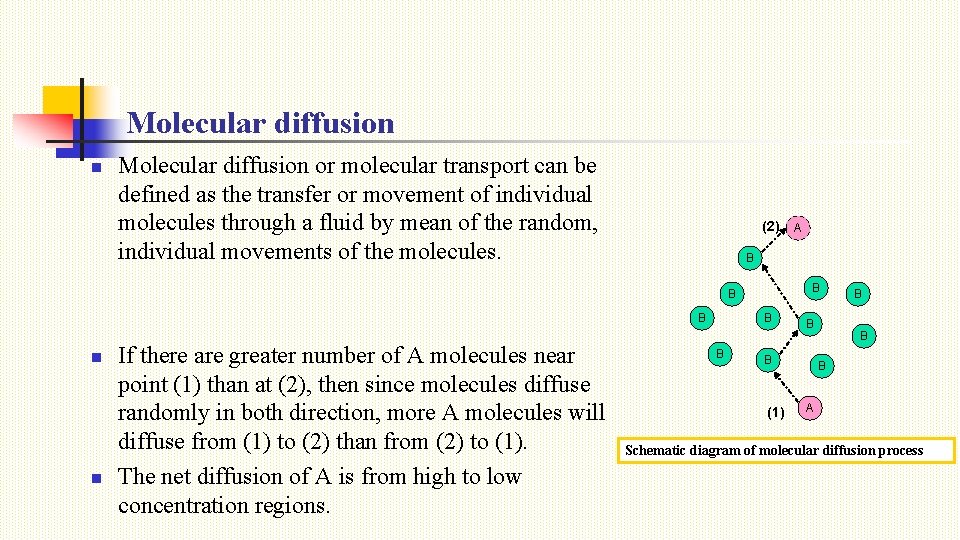
Molecular diffusion n Molecular diffusion or molecular transport can be defined as the transfer or movement of individual molecules through a fluid by mean of the random, individual movements of the molecules. (2) B B n n If there are greater number of A molecules near point (1) than at (2), then since molecules diffuse randomly in both direction, more A molecules will diffuse from (1) to (2) than from (2) to (1). The net diffusion of A is from high to low concentration regions. A B B (1) B B B A Schematic diagram of molecular diffusion process

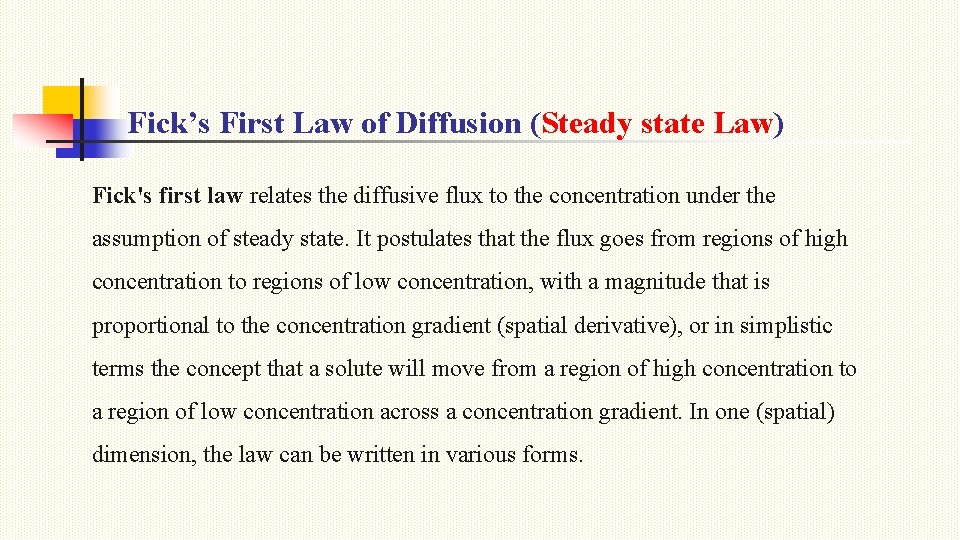
Fick’s First Law of Diffusion (Steady state Law) Fick's first law relates the diffusive flux to the concentration under the assumption of steady state. It postulates that the flux goes from regions of high concentration to regions of low concentration, with a magnitude that is proportional to the concentration gradient (spatial derivative), or in simplistic terms the concept that a solute will move from a region of high concentration to a region of low concentration across a concentration gradient. In one (spatial) dimension, the law can be written in various forms.

Assumptions of Fick’s First Law 1 - Binary system 2 - Ideal gas mixture 3 - Steady state 4 - One dimensional 5 - Unit area 6 - Constant total concentration 7 - Constant total volume 8 - Constant total pressure 9 - Constant temperature 10 -No chemical reaction

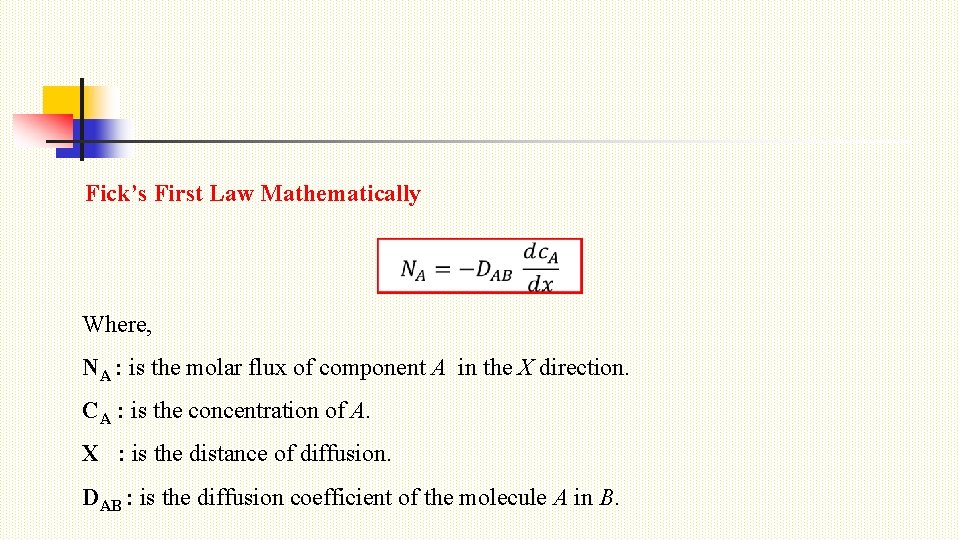
Fick’s First Law Mathematically Where, NA : is the molar flux of component A in the X direction. CA : is the concentration of A. X : is the distance of diffusion. DAB : is the diffusion coefficient of the molecule A in B.
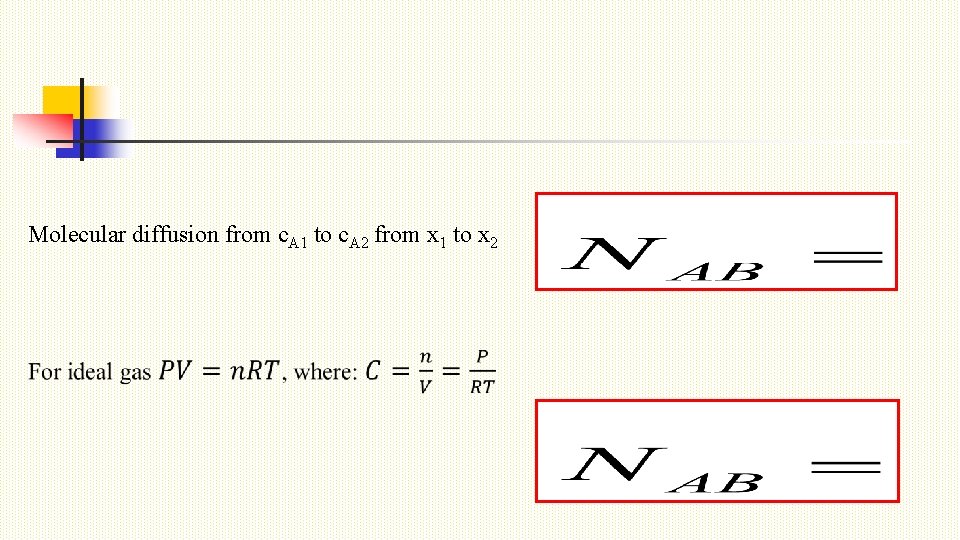
Molecular diffusion from c. A 1 to c. A 2 from x 1 to x 2

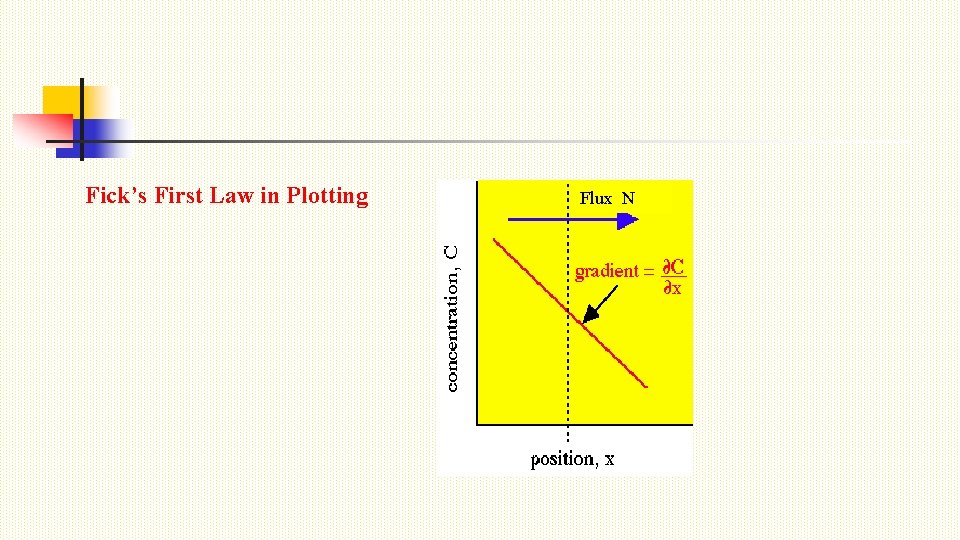
Fick’s First Law in Plotting Flux N

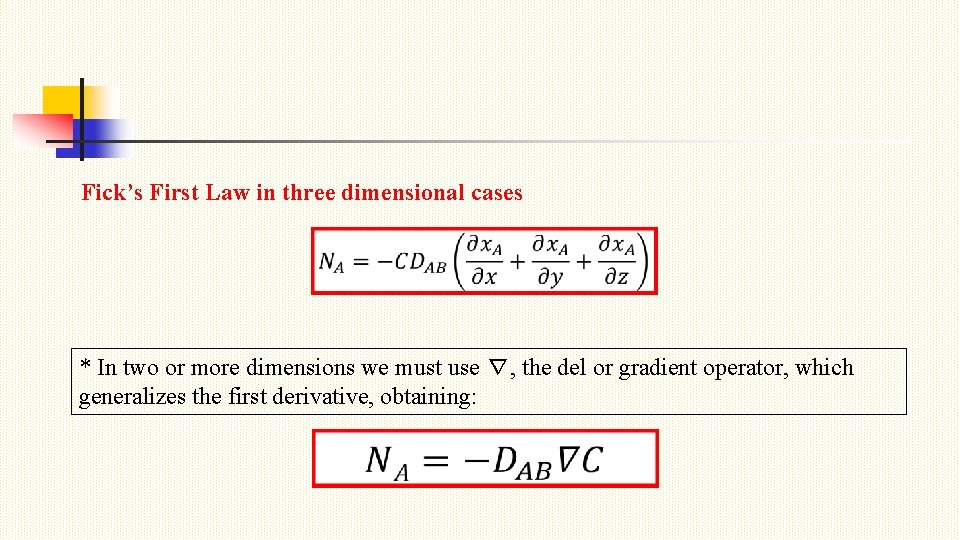
Fick’s First Law in three dimensional cases * In two or more dimensions we must use ∇, the del or gradient operator, which generalizes the first derivative, obtaining:

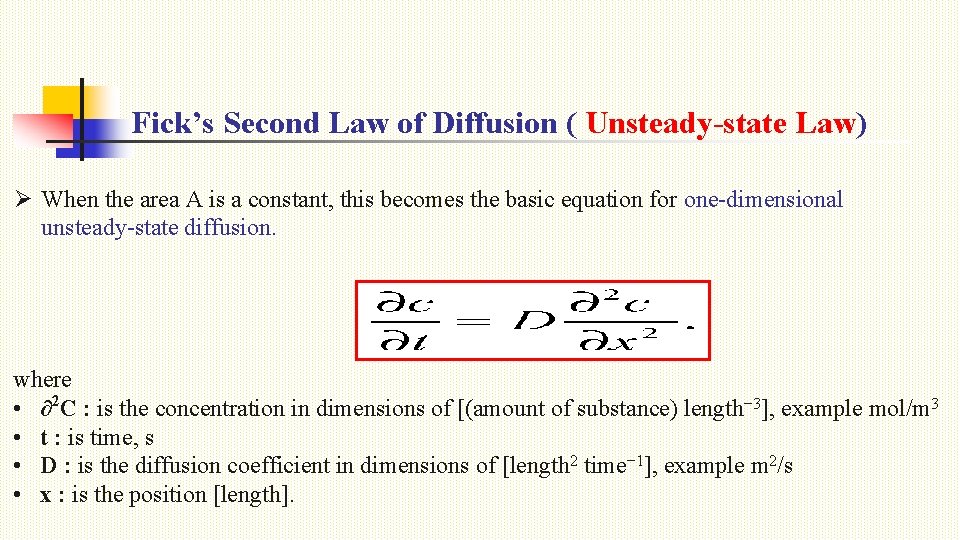
Fick’s Second Law of Diffusion ( Unsteady-state Law) Ø When the area A is a constant, this becomes the basic equation for one-dimensional unsteady-state diffusion. where • ∂2 C : is the concentration in dimensions of [(amount of substance) length− 3], example mol/m 3 • t : is time, s • D : is the diffusion coefficient in dimensions of [length 2 time− 1], example m 2/s • x : is the position [length].

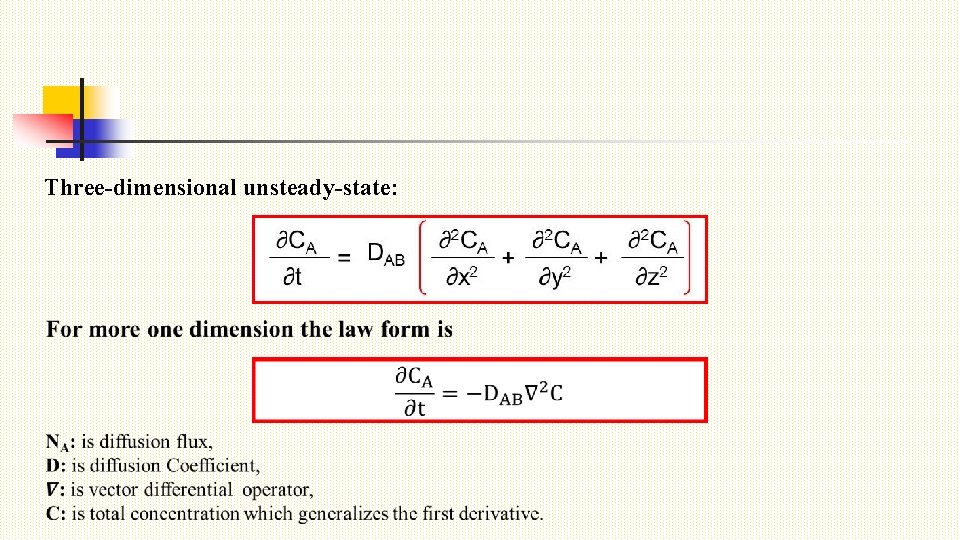
Three-dimensional unsteady-state:

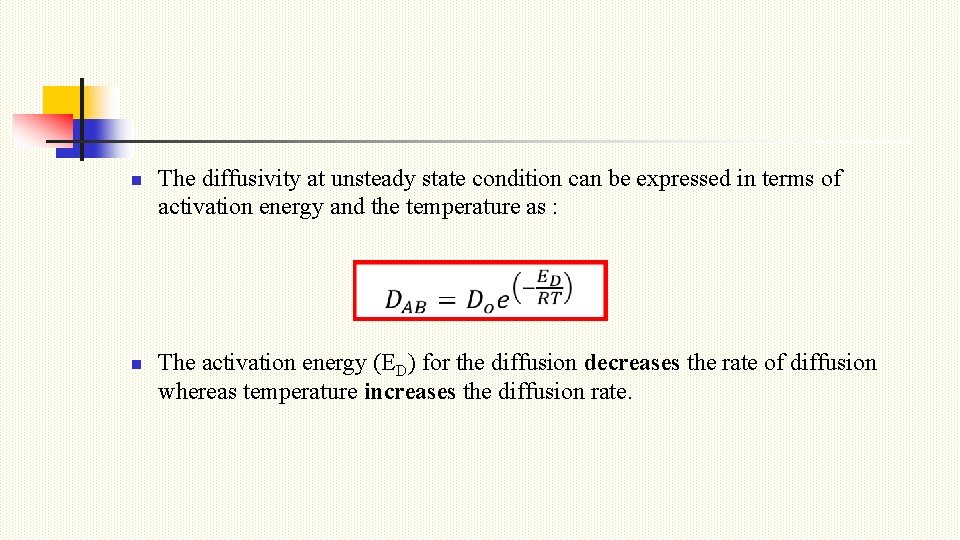
n n The diffusivity at unsteady state condition can be expressed in terms of activation energy and the temperature as : The activation energy (ED) for the diffusion decreases the rate of diffusion whereas temperature increases the diffusion rate.

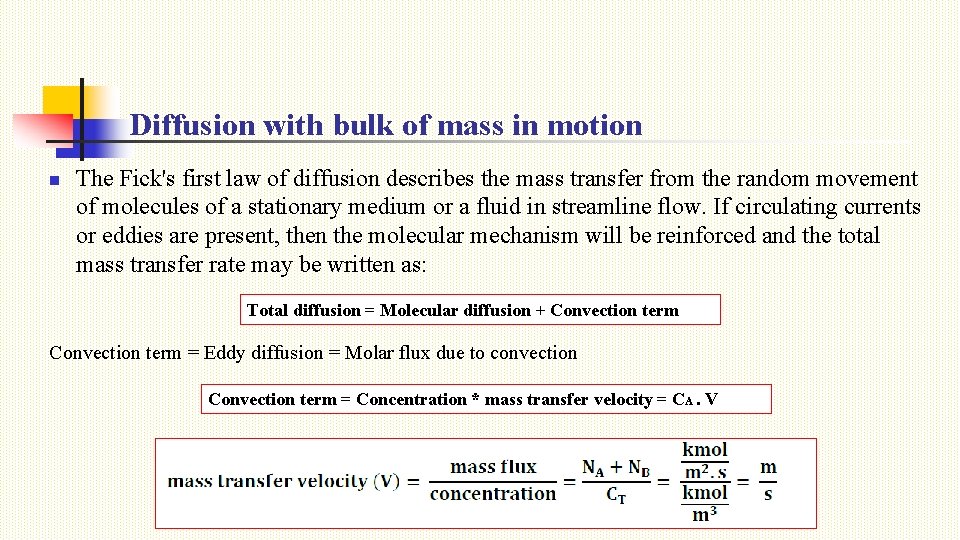
Diffusion with bulk of mass in motion n The Fick's first law of diffusion describes the mass transfer from the random movement of molecules of a stationary medium or a fluid in streamline flow. If circulating currents or eddies are present, then the molecular mechanism will be reinforced and the total mass transfer rate may be written as: Total diffusion = Molecular diffusion + Convection term = Eddy diffusion = Molar flux due to convection Convection term = Concentration * mass transfer velocity = CA. V

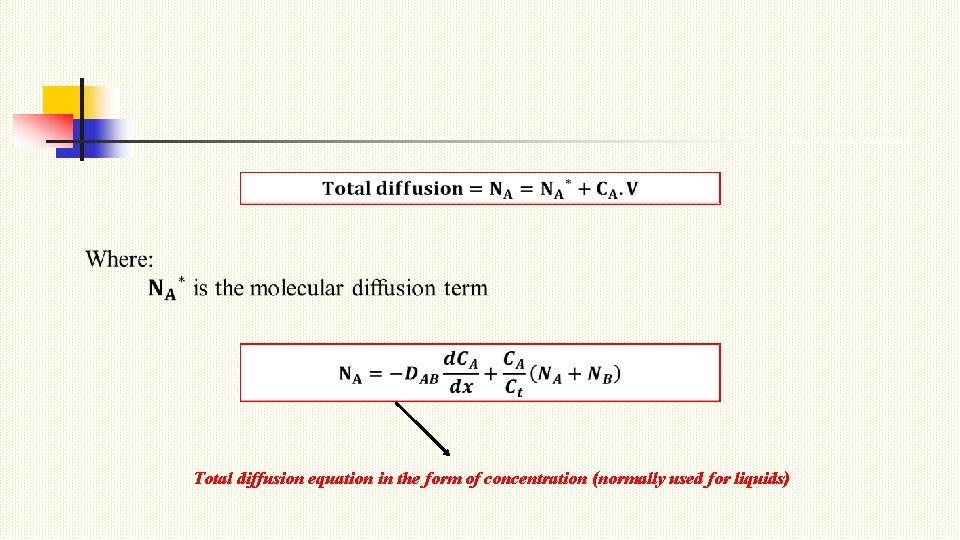
Total diffusion equation in the form of concentration (normally used for liquids)

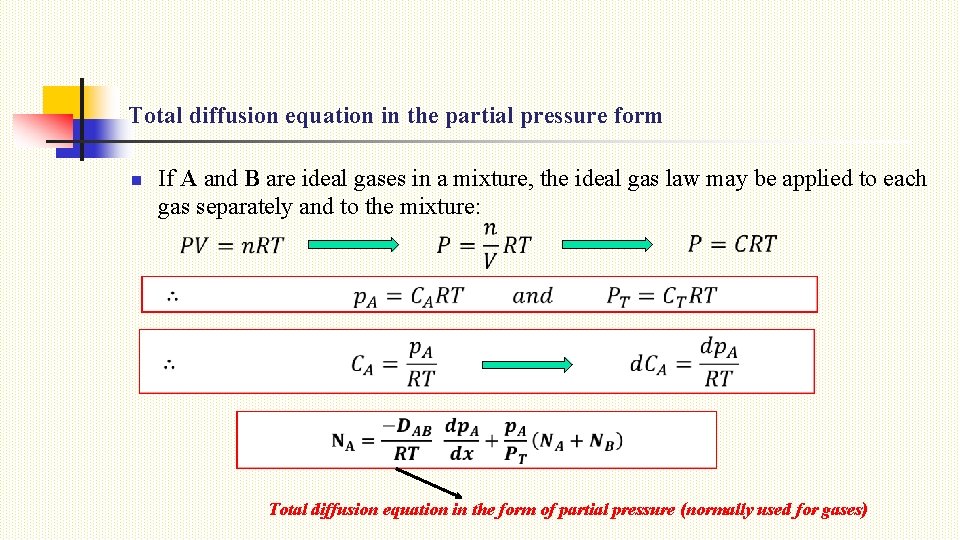
Total diffusion equation in the partial pressure form n If A and B are ideal gases in a mixture, the ideal gas law may be applied to each gas separately and to the mixture: Total diffusion equation in the form of partial pressure (normally used for gases)

T h a n k Y o u
 Feup university of porto
Feup university of porto Ulfg2
Ulfg2 Clemson ece
Clemson ece Faculty of mechanical engineering thammasat university
Faculty of mechanical engineering thammasat university Nit calicut chemistry department faculty
Nit calicut chemistry department faculty Department of information engineering university of padova
Department of information engineering university of padova Department of information engineering university of padova
Department of information engineering university of padova University of sargodha engineering department
University of sargodha engineering department Chemical engineering wisconsin
Chemical engineering wisconsin Chemical engineering university of cincinnati
Chemical engineering university of cincinnati University of cincinnati chemical engineering
University of cincinnati chemical engineering Syracuse university chemical engineering
Syracuse university chemical engineering University of split faculty of maritime studies
University of split faculty of maritime studies University of bridgeport computer science
University of bridgeport computer science University of bridgeport computer science
University of bridgeport computer science Hubert kairuki memorial university faculty of medicine
Hubert kairuki memorial university faculty of medicine Semmelweis
Semmelweis Applied medical sciences
Applied medical sciences Computer science fsu
Computer science fsu Mendel university faculty of business and economics
Mendel university faculty of business and economics Singularity executive program
Singularity executive program







































