Minia University Faculty of Engineering Chemical Engineering Department









- Slides: 26

Minia University Faculty of Engineering Chemical Engineering Department Course Title: Unit Operation II Course Code: CHE 321 Third Year Course Coordinator: Prof. Dr. Mohammad Shawky Lecture No. 7

Physico-Chemical Studies on Adsorbent Physical Analysis: (1) Pore size distribution Porosity (4) Total pore volume (5) Particle shape (6) Moisture content (7) PH determination (8)Total surface area

Physico-Chemical Studies on Adsorbent Physical Analysis: (1) Pore size distribution 3) Porosity (4) Total pore volume (5) Particle shape (6) Moisture content (7) PH determination (8)Total surface area

Physico-Chemical Studies on Adsorbent Porosity Particle porosity Bed porosity

Physico-Chemical Studies on Adsorbent Porosity Particle porosity Bed porosity

Physico-Chemical Studies on Adsorbent (3) Porosity : Types of porosity: 1. Particle porosity (εp): εp= fraction of internal voids = particle porosity =voidage

Physico-Chemical Studies on Adsorbent Porosity Particle porosity Bed porosity

Physico-Chemical Studies on Adsorbent Porosity Particle porosity Bed porosity

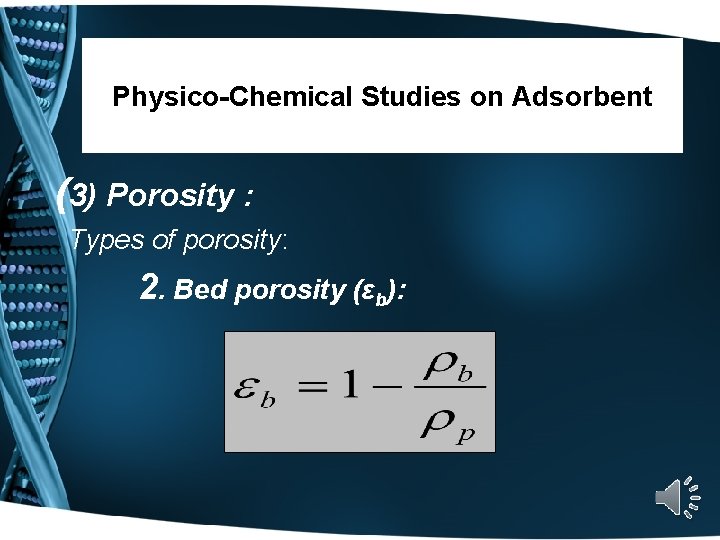
Physico-Chemical Studies on Adsorbent (3) Porosity : Types of porosity: 2. Bed porosity (εb):

Physico-Chemical Studies on Adsorbent 2. Bed Porosity (εb): Porosity can be measured using Mercury Porosimetry, for pith initially dried in a vacuum at 50ºC for 8 hr. The surface area of the pith was measured on Y uasa surface area apparatus (BET method). Pore volumes and average pore radius of the pith was determined on a Carlo-Erba mercury porosimater (Model 1520).

Physico-Chemical Studies on Adsorbent Physical Analysis: (1) Pore size distribution 3) Porosity (4) Total pore volume (5) Particle shape (6) Moisture content (7) PH determination (8)Total surface area

2. 1 Physico-Chemical Studies on Adsorbent Physical Analysis: (1) Pore size distribution Porosity (4) Total pore volume (5) Particle shape (6) Moisture content (7) PH determination (8)Total surface area

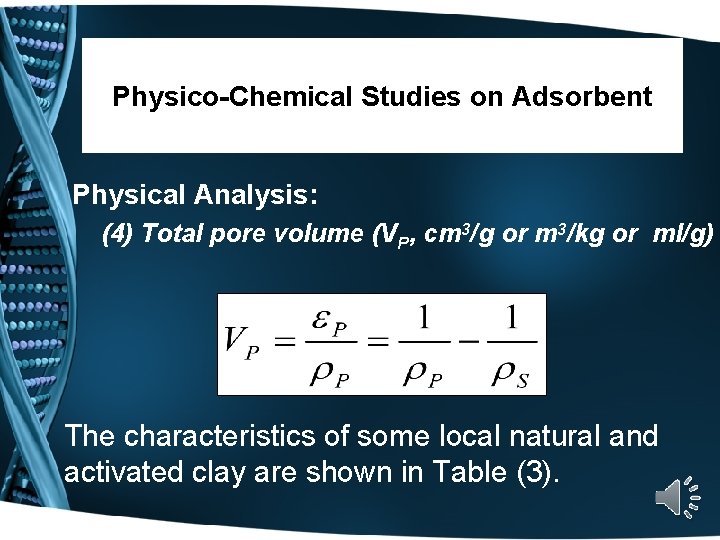
Physico-Chemical Studies on Adsorbent Physical Analysis: (4) Total pore volume (VP, cm 3/g or m 3/kg or ml/g) The characteristics of some local natural and activated clay are shown in Table (3).

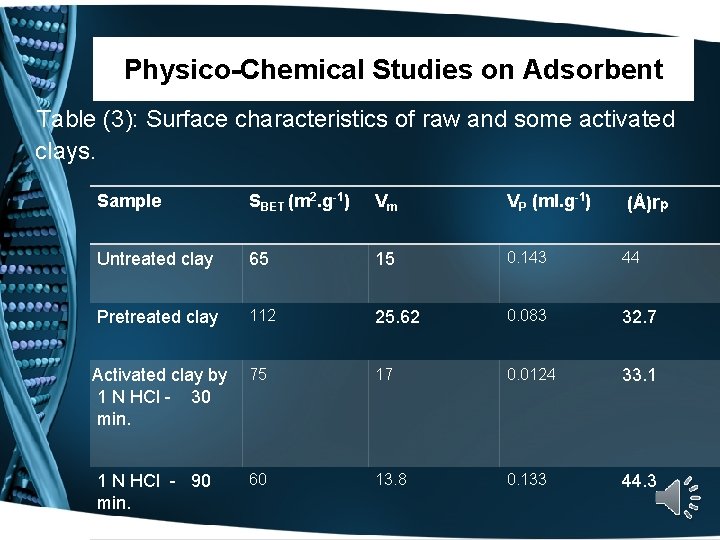
Physico-Chemical Studies on Adsorbent Table (3): Surface characteristics of raw and some activated clays. Sample SBET (m 2. g-1) Vm VP (ml. g-1) Untreated clay 65 15 0. 143 44 Pretreated clay 112 25. 62 0. 083 32. 7 Activated clay by 1 N HCl - 30 min. 75 17 0. 0124 33. 1 60 13. 8 0. 133 44. 3 1 N HCl - 90 min. (Å)rp

Physico-Chemical Studies on Adsorbent Table (3): Surface characteristics of raw and some activated clays. Sample SBET (m 2. g-1) Vm VP (ml. g-1) 1 N HCl - 90 min. 60 13. 8 0. 133 44. 3 1 N HCl - 180 min 40 9 0. 107 53. 5 25 0. 314 57. 6 2 N HCl - 30 min. 109 (Å)rp 2 N HCl - 90 min 115 26. 34 0. 326 56. 7 2 N HCl - 180 min 127 29. 0 0. 278 43. 8

Physico-Chemical Studies on Adsorbent Physical Analysis: (1) Pore size distribution ) Porosity (4) Total pore volume (5) Particle shape (6) Moisture content (7) PH determination (8)Total surface area

Physico-Chemical Studies on Adsorbent Physical Analysis: (1) Pore size distribution Porosity (4) Total pore volume (5) Particle shape (6) Moisture content (7) PH determination (8)Total surface area

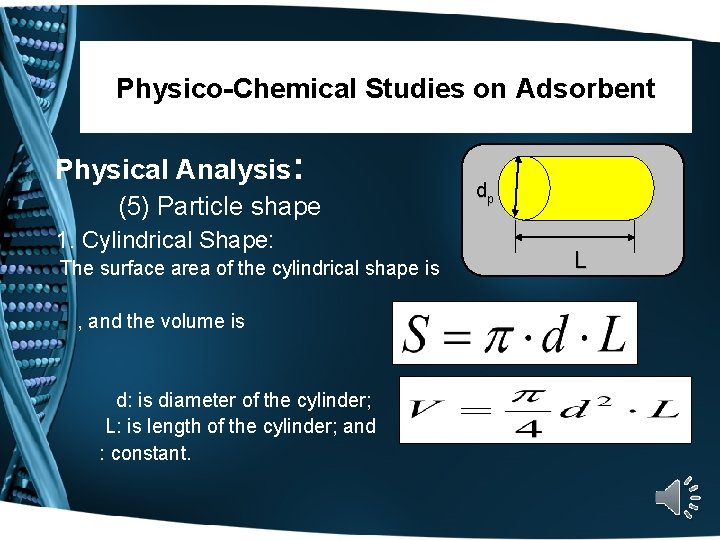
Physico-Chemical Studies on Adsorbent Physical Analysis: (5) Particle shape Adsorbent may be: a) Powder form--------Cylindrical Shape b) Granular form-------Spherical Shape

Physico-Chemical Studies on Adsorbent Physical Analysis: (5) Particle shape 1. Cylindrical Shape: The surface area of the cylindrical shape is , and the volume is d: is diameter of the cylinder; L: is length of the cylinder; and : constant. dp L

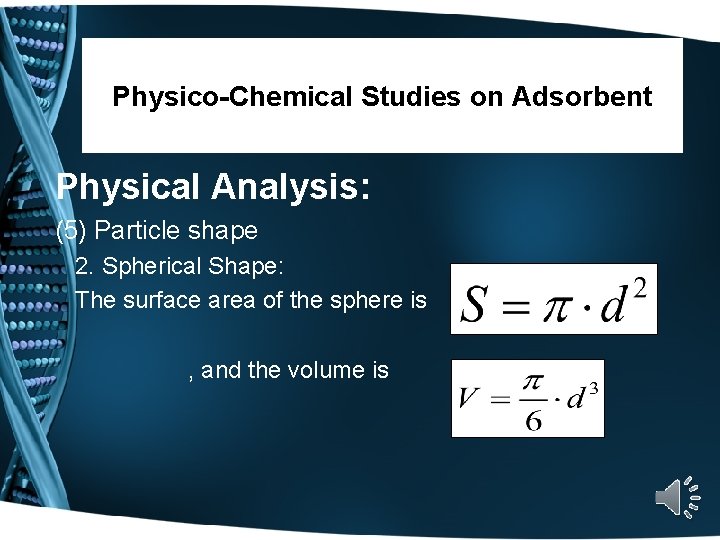
Physico-Chemical Studies on Adsorbent Physical Analysis: (5) Particle shape 2. Spherical Shape: The surface area of the sphere is , and the volume is

Physico-Chemical Studies on Adsorbent Physical Analysis: (1) Pore size distribution Porosity (4) Total pore volume (5) Particle shape (6) Moisture content (7) PH determination (8)Total surface area

2. 1 Physico-Chemical Studies on Adsorbent Physical Analysis: (1) Pore size distribution Porosity (4) Total pore volume (5) Particle shape (6) Moisture content (7) PH determination (8)Total surface area

Physico-Chemical Studies on Adsorbent Physical Analysis: (6) Moisture content Under humid conditions, adsorbents may adsorb perhaps 25 to 30% moisture, over a few months, and still appear dry. For many purposes, particularly in wastewater treatment, the moisture content does not adversely affect the adsorbent performance, but merely act as a dilute, so that an additional quantity of adsorbent is required to provide the desired dry weight. Moister content of local natural clay = 7. 9 ± 0. 25% measured after sample dried at 105 ºC for 12 hours.

Physico-Chemical Studies on Adsorbent Physical Analysis: (1) Pore size distribution ) Porosity (4) Total pore volume (5) Particle shape (6) Moisture content (7) PH determination (8)Total surface area

2. 1 Physico-Chemical Studies on Adsorbent Physical Analysis: (1) Pore size distribution Porosity (4) Total pore volume (5) Particle shape (6) Moisture content (7) PH determination (8)Total surface area

Physico-Chemical Studies on Adsorbent Physical Analysis: (7) PH determination For natural clay PH measured by the following procedure: 1. Suspension containing 1 g of natural clay in 25 ml of distilled water. 2. After 48 h aging, the PH values of different clay suspensions were measured by means of accurate PH meter.
 Faculty of engineering university of porto
Faculty of engineering university of porto Lebanese university faculty of engineering
Lebanese university faculty of engineering Clemson ece lab manual
Clemson ece lab manual Faculty of mechanical engineering thammasat university
Faculty of mechanical engineering thammasat university Nit calicut chemistry department
Nit calicut chemistry department Department of information engineering university of padova
Department of information engineering university of padova Information engineering padova
Information engineering padova University of sargodha engineering department
University of sargodha engineering department University of wisconsin madison chemical engineering
University of wisconsin madison chemical engineering Cincinnati chemical engineering
Cincinnati chemical engineering University of cincinnati chemical engineering
University of cincinnati chemical engineering Syracuse university chemical engineering
Syracuse university chemical engineering University of split faculty of maritime studies
University of split faculty of maritime studies University of bridgeport computer science
University of bridgeport computer science Bridgeport university computer science
Bridgeport university computer science Hubert kairuki memorial university faculty of medicine
Hubert kairuki memorial university faculty of medicine Semmelweis university faculty of medicine
Semmelweis university faculty of medicine Applied medical sciences
Applied medical sciences Computer science fsu
Computer science fsu Mendel university - faculty of business and economics
Mendel university - faculty of business and economics Singularity university faculty
Singularity university faculty Agnes csaki semmelweis
Agnes csaki semmelweis Masaryk university medical faculty
Masaryk university medical faculty Ldap cuni
Ldap cuni Faculty of veterinary medicine cairo university logo
Faculty of veterinary medicine cairo university logo Faculty of law of the university of zagreb
Faculty of law of the university of zagreb University of montenegro faculty of law
University of montenegro faculty of law


















































