Minia University Faculty of Engineering Chemical Engineering Department




- Slides: 16

Minia University Faculty of Engineering Chemical Engineering Department Course Title: Mass Transfer Course Code: CHE 313 Third Year Course Coordinator: P r o f. D r. M o h a m m a d S h a w k y Lecture No. 8 DIFFUSIVITY for LIQUIDS

Contents n What is Diffusivity? n Determination of Diffusivity of Liquids : By Diaphragm Cell Method n Prediction of Diffusivity : 1. Stokes – Einstein Correlation (1905) 2. Wilke-Chang Correlation (1955) 3. Hayduk – Laudie Correlation (1974) 4. Hayduk – Minhas Correlation (1982) n Effect of Some Variables on Diffusivity of Liquids

What is Diffusivity? n The proportionality factor of Fick’s law is called diffusivity or diffusion coefficient which can be defined as the ratio of the flux to its concentration gradient out-of-plane. n Dimensions: L 2 / θ n Units (SI): m 2 / s

Determination of Diffusivity in Liquids n Here, a certain value of diffusivity is obtained by, 1 - Practical Methods (Experimental – Literature) 2 - Prediction Methods n In a prediction method, we use correlations or empirical correlations which have no mathematical derivation.

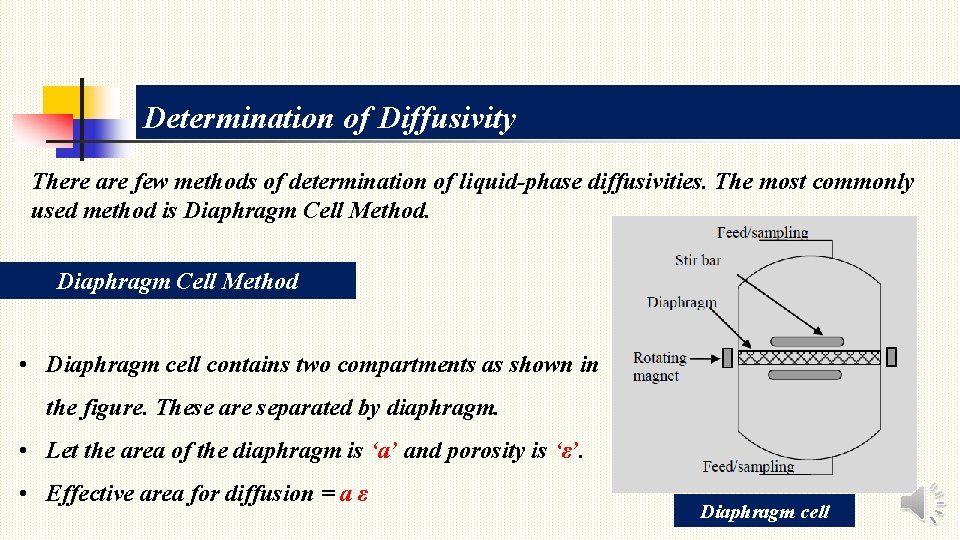
Determination of Diffusivity There are few methods of determination of liquid-phase diffusivities. The most commonly used method is Diaphragm Cell Method • Diaphragm cell contains two compartments as shown in the figure. These are separated by diaphragm. • Let the area of the diaphragm is ‘a’ and porosity is ‘ε’. • Effective area for diffusion = a ε Diaphragm cell

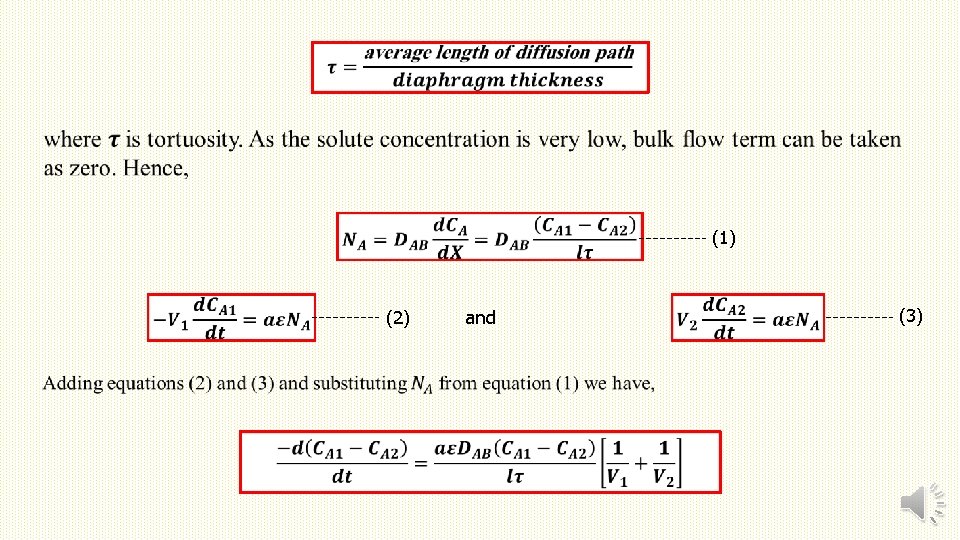
(1) (2) and (3)

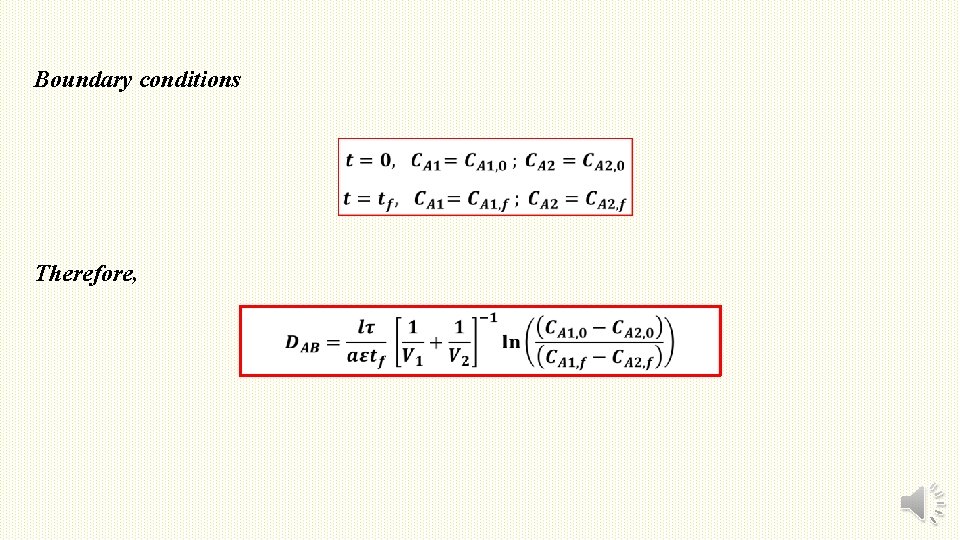
Boundary conditions Therefore,

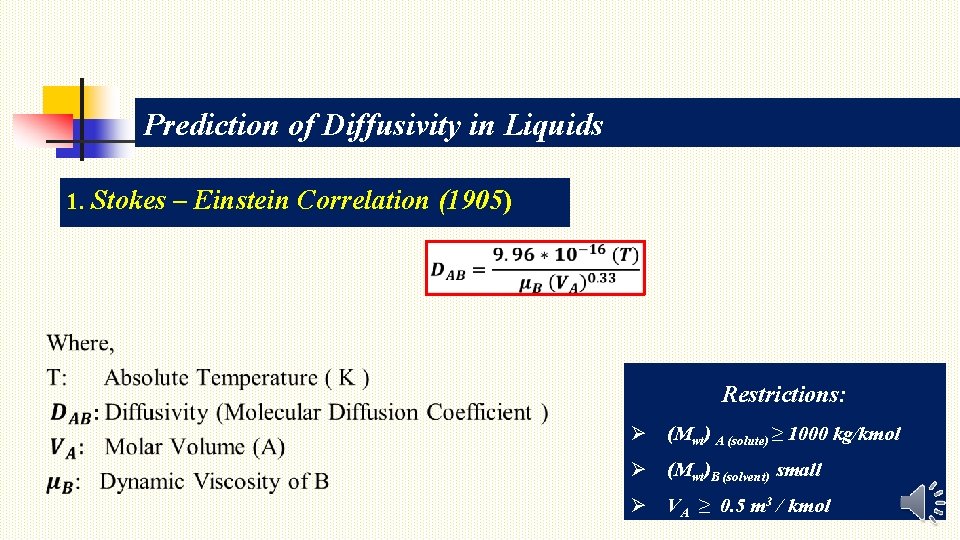
Prediction of Diffusivity in Liquids 1. Stokes – Einstein Correlation (1905) Restrictions: Ø (Mwt) A (solute) ≥ 1000 kg/kmol Ø (Mwt)B (solvent) small Ø VA ≥ 0. 5 m 3 / kmol

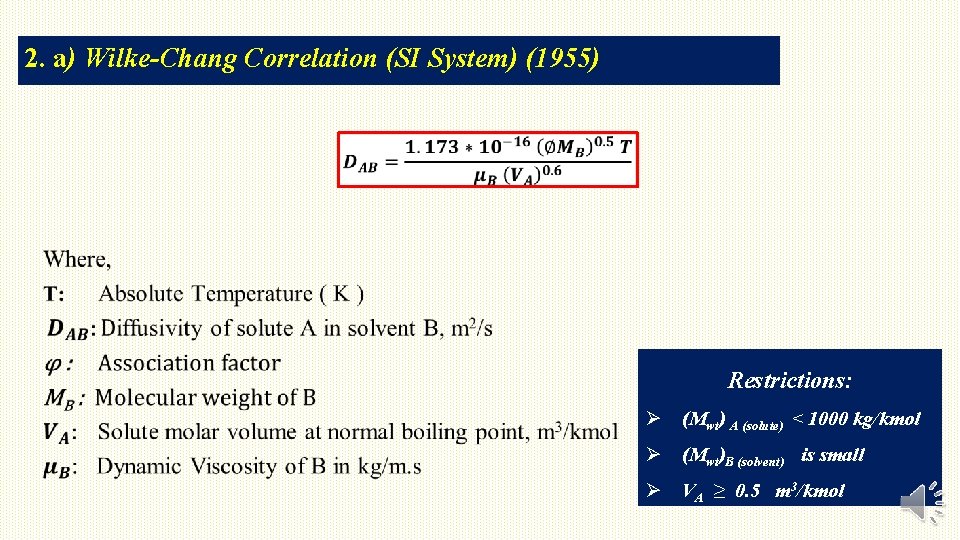
2. a) Wilke-Chang Correlation (SI System) (1955) Restrictions: Ø (Mwt) A (solute) < 1000 kg/kmol Ø (Mwt)B (solvent) is small Ø VA ≥ 0. 5 m 3/kmol

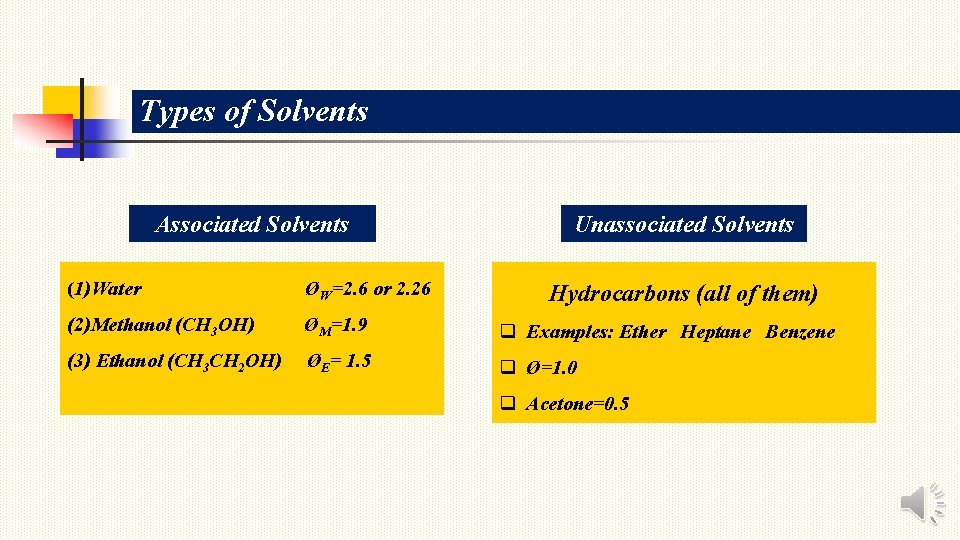
Types of Solvents Associated Solvents Unassociated Solvents (1)Water ØW=2. 6 or 2. 26 (2)Methanol (CH 3 OH) ØM=1. 9 q Examples: Ether Heptane Benzene (3) Ethanol (CH 3 CH 2 OH) ØE= 1. 5 q Ø=1. 0 Hydrocarbons (all of them) q Acetone=0. 5

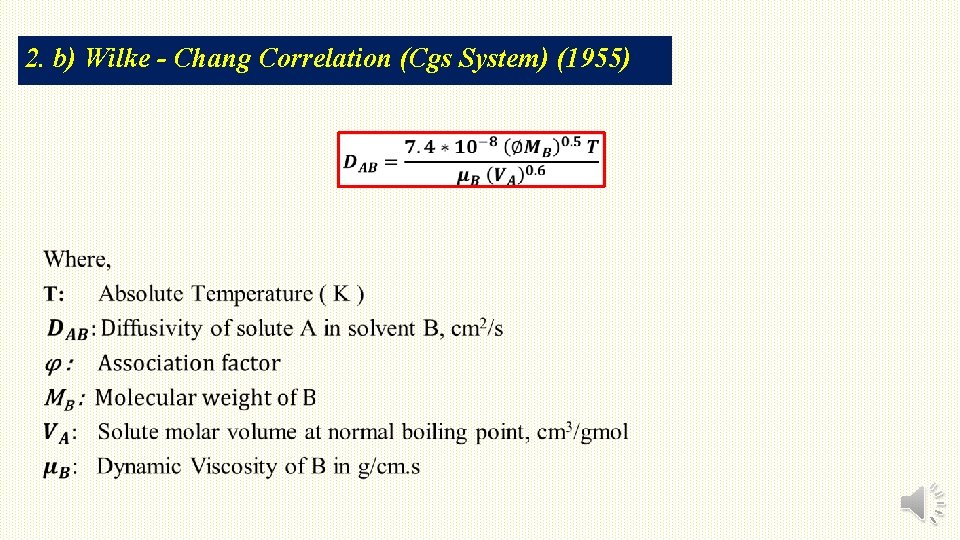
2. b) Wilke - Chang Correlation (Cgs System) (1955)

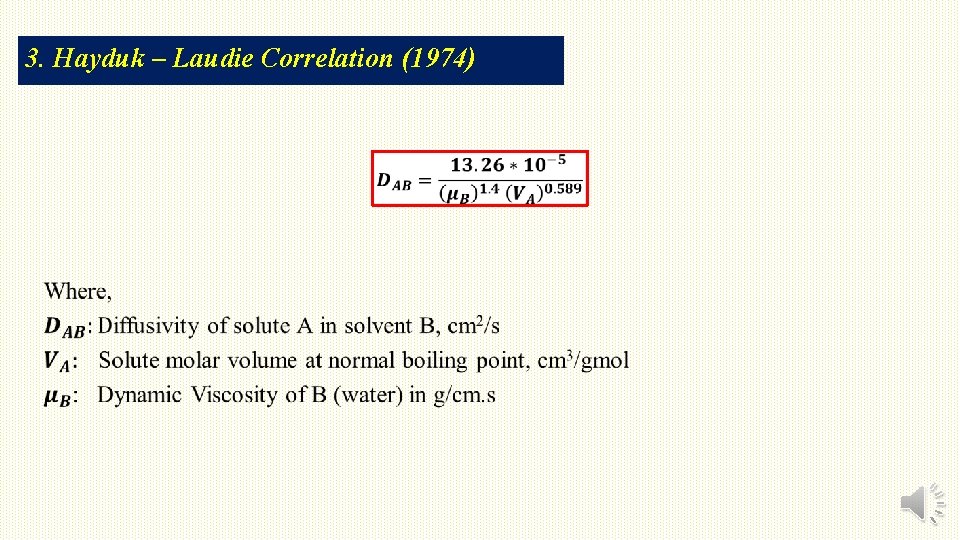
3. Hayduk – Laudie Correlation (1974)

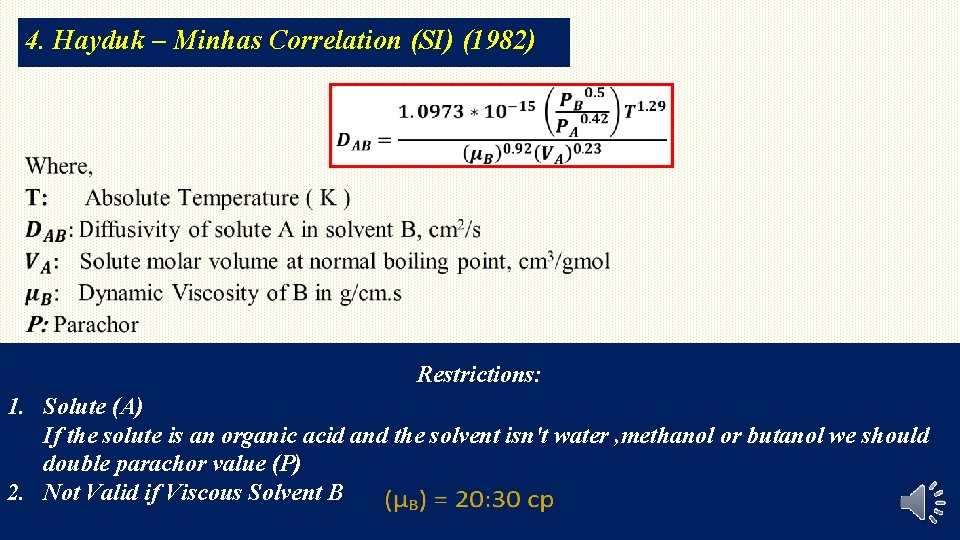
4. Hayduk – Minhas Correlation (SI) (1982) Restrictions: 1. Solute (A) If the solute is an organic acid and the solvent isn't water , methanol or butanol we should double parachor value (P) 2. Not Valid if Viscous Solvent B

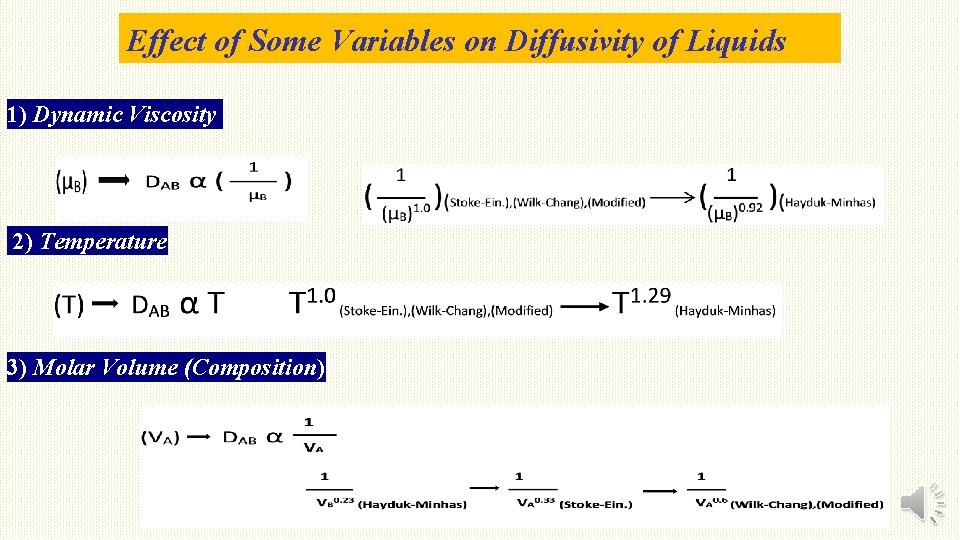
Effect of Some Variables on Diffusivity of Liquids 1) Dynamic Viscosity 2) Temperature 3) Molar Volume (Composition)

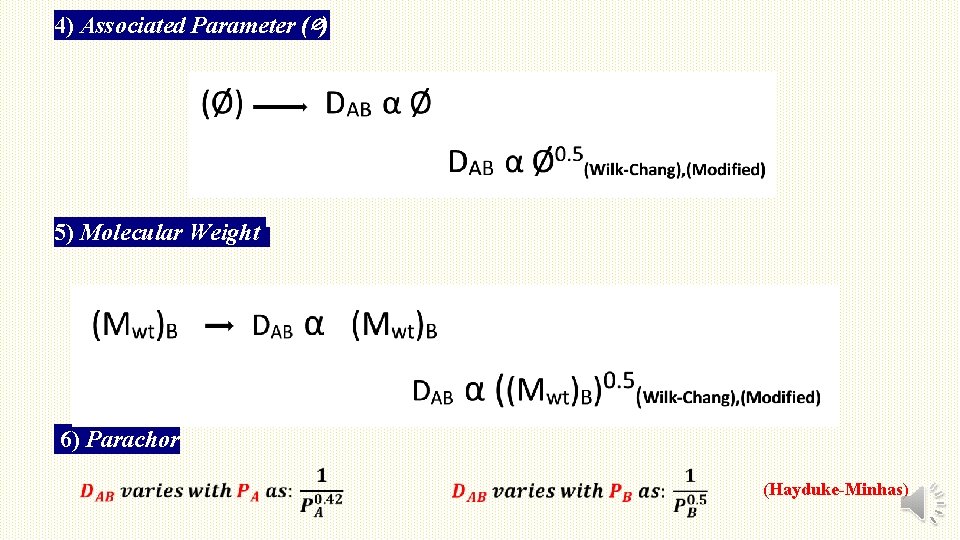
4) Associated Parameter (∅) 5) Molecular Weight 6) Parachor (Hayduke-Minhas)

T h a n k Y o u
 Feup university of porto
Feup university of porto Faculty of engineering lebanese university
Faculty of engineering lebanese university Ece clemson
Ece clemson Faculty of mechanical engineering thammasat university
Faculty of mechanical engineering thammasat university Nit calicut chemistry department faculty
Nit calicut chemistry department faculty Department of information engineering university of padova
Department of information engineering university of padova Department of information engineering university of padova
Department of information engineering university of padova University of sargodha engineering department
University of sargodha engineering department Chemical engineering wisconsin
Chemical engineering wisconsin Cincinnati chemical engineering
Cincinnati chemical engineering University of cincinnati chemical engineering
University of cincinnati chemical engineering Syracuse university chemical engineering
Syracuse university chemical engineering Herszon kherson maritime college of merchant marine fleet
Herszon kherson maritime college of merchant marine fleet University of bridgeport computer science faculty
University of bridgeport computer science faculty University of bridgeport engineering
University of bridgeport engineering Hubert kairuki memorial university faculty of medicine
Hubert kairuki memorial university faculty of medicine Solid thyroid nodule
Solid thyroid nodule































