Minia University Faculty of Engineering Chemical Engineering Department








- Slides: 15

Minia University Faculty of Engineering Chemical Engineering Department Classifications of Polymers By Prof. Wael Abdelmoez Professor of Environment & Energy

Addition Polymers The polymers formed by the addition of monomers repeatedly without removal of by products are called addition polymers. These polymers contains all the atoms of monomers hence their molecular weight are integral multiple of monomer unit. E. g. Teflon, Polyethylene, Polypropylene, PVC.

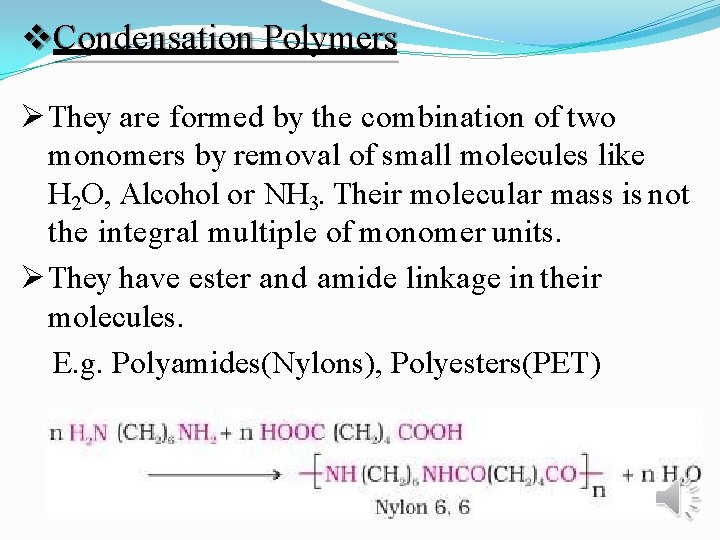
Condensation Polymers They are formed by the combination of two monomers by removal of small molecules like H 2 O, Alcohol or NH 3. Their molecular mass is not the integral multiple of monomer units. They have ester and amide linkage in their molecules. E. g. Polyamides(Nylons), Polyesters(PET)

Linear Polymers In these polymers monomers are linked with each other and form a long straight chain. These chains has no any side chains. Their molecules are closely packed and have high density, tensile strength, and melting point. E. g. HDPE, Nylons

Branched Polymers They have a straight long chain with different side chains. Their molecules are irregularly packed hence they have low density, Tensile strength and melting point. E. g. LDPE, LLDPE

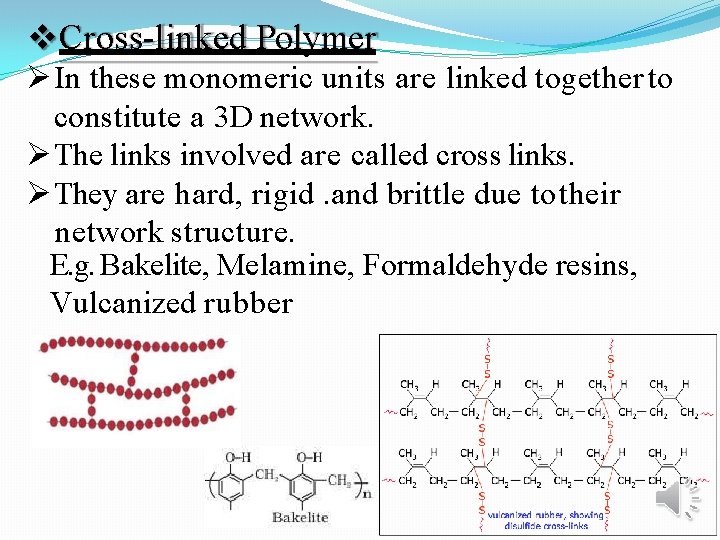
Cross-linked Polymer In these monomeric units are linked together to constitute a 3 D network. The links involved are called cross links. They are hard, rigid. and brittle due to their network structure. E. g. Bakelite, Melamine, Formaldehyde resins, Vulcanized rubber


Fibers If polymer is drawn into long filament like materiel whose length is at least 100 times it’s diameter, are said to be converted into fiber. They have high tensile strength because of high intermolecular attractive force like Hydrogen bonding. Highly crystalline. E. g. Nylon, Terylene.

Plastics Polymer is shaped into hard and tough utility articles by application of heat and pressure, is known as plastics. Here the intermolecular force between polymeric chains are intermediate between elastomers and fibers. They are partially crystalline. E. g. Polystyrene, PVC, PMMA

Elastomers They are solids with rubber like elastic properties. Here the polymeric chains are held together by the weakest intermolecular forces so they are highly amorphous in nature. These weak binding forces permit them to be stretched. E. g. Natural rubber, BUNA-S, BUNA-N, Vulcanised rubber


Resins Low molecular weight. Polymers which are used as adhesives, sealants etc. , in a liquid form are described as liquid resins. E. g. Epoxy adhesives and polysulphides sealants.

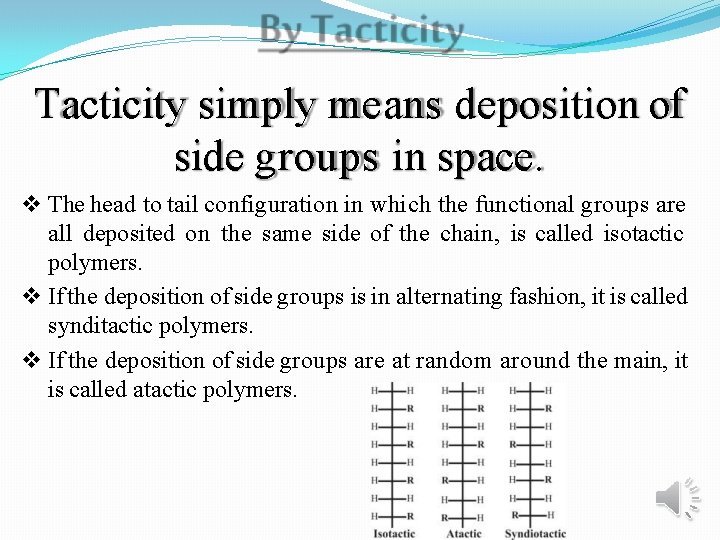
Tacticity simply means deposition of side groups in space. The head to tail configuration in which the functional groups are all deposited on the same side of the chain, is called isotactic polymers. If the deposition of side groups is in alternating fashion, it is called synditactic polymers. If the deposition of side groups are at random around the main, it is called atactic polymers.

By Crystallinity 1. Crystalline – Monomers arranged in ordered way. 2. Amorphous – Monomers arranged in random way. By Backbone Atom 1. Organic – Polymer Backbone is made-up of carbon atom. 2. Inorganic – Polymer Backbone is made-up of other atoms

 Faculty of engineering university of porto
Faculty of engineering university of porto Ulfg2
Ulfg2 Clemson university electrical engineering faculty
Clemson university electrical engineering faculty Faculty of mechanical engineering thammasat university
Faculty of mechanical engineering thammasat university Keralastec
Keralastec Department of information engineering university of padova
Department of information engineering university of padova Information engineering padova
Information engineering padova University of sargodha engineering department
University of sargodha engineering department University of wisconsin madison chemical engineering
University of wisconsin madison chemical engineering Cincinnati chemical engineering
Cincinnati chemical engineering University of cincinnati chemical engineering
University of cincinnati chemical engineering Syracuse university chemical engineering
Syracuse university chemical engineering University of split faculty of maritime studies
University of split faculty of maritime studies University of bridgeport computer science
University of bridgeport computer science University of bridgeport computer science
University of bridgeport computer science Hubert kairuki memorial university faculty of medicine
Hubert kairuki memorial university faculty of medicine





























