MICROWAVE STRUCTURE FOR THE PROPIOLIC ACID FORMIC ACID
- Slides: 17

MICROWAVE STRUCTURE FOR THE PROPIOLIC ACID – FORMIC ACID COMPLEX† STEVE KUKOLICH, ERIK MITCHELL╬ , SPENCER CAREY, MING SUN, AND BRYAN SARGUS, Dept. of Chemistry and Biochemistry, The University of Arizona, Tucson, Arizona 85721. ╬ Present address: Patrick Air Force Base, 32925, United States † This material is based on work supported by the National Science Foundation under Grant Nos. CHE-0721505, CHE 0809053 and CHE-10557796 OSU – June – 2013 - SGK 1

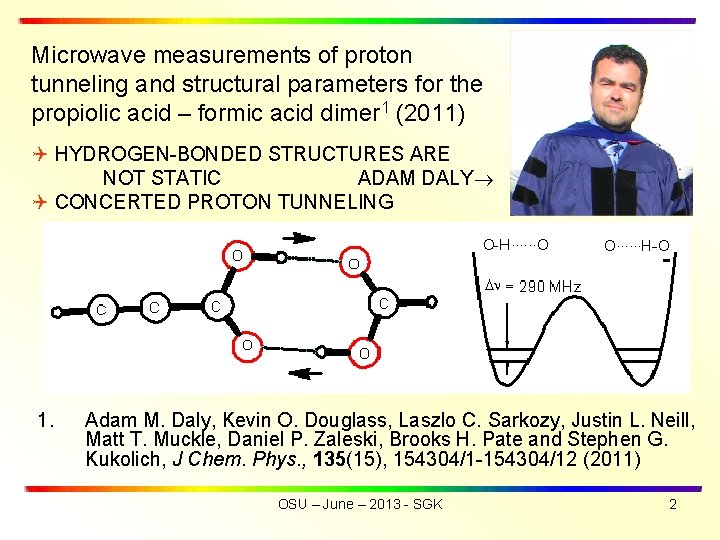
Microwave measurements of proton tunneling and structural parameters for the propiolic acid – formic acid dimer 1 (2011) HYDROGEN-BONDED STRUCTURES ARE NOT STATIC ADAM DALY CONCERTED PROTON TUNNELING 1. Adam M. Daly, Kevin O. Douglass, Laszlo C. Sarkozy, Justin L. Neill, Matt T. Muckle, Daniel P. Zaleski, Brooks H. Pate and Stephen G. Kukolich, J Chem. Phys. , 135(15), 154304/1 -154304/12 (2011) OSU – June – 2013 - SGK 2

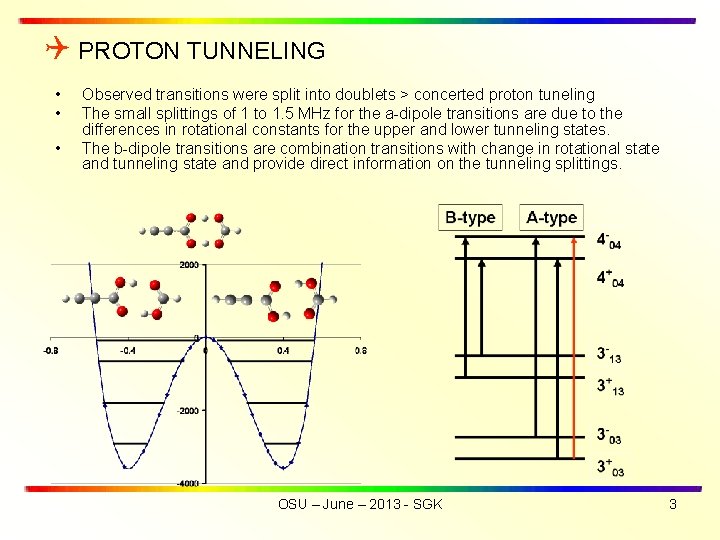
PROTON TUNNELING • • • Observed transitions were split into doublets > concerted proton tuneling The small splittings of 1 to 1. 5 MHz for the a-dipole transitions are due to the differences in rotational constants for the upper and lower tunneling states. The b-dipole transitions are combination transitions with change in rotational state and tunneling state and provide direct information on the tunneling splittings. OSU – June – 2013 - SGK 3

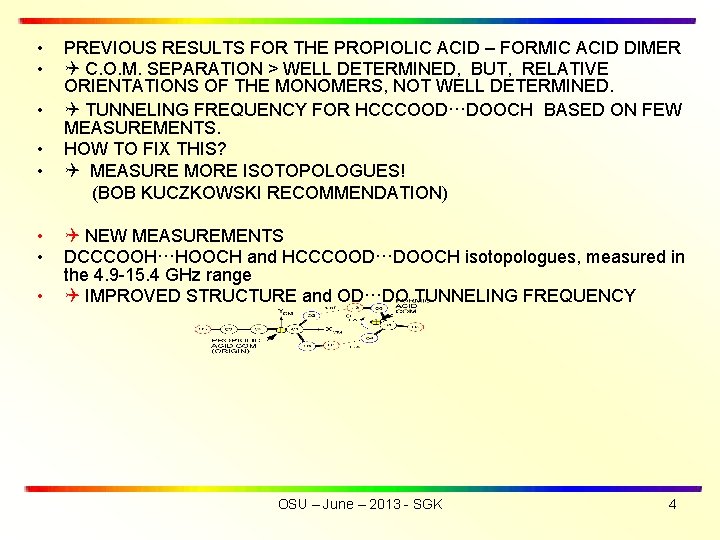
• • PREVIOUS RESULTS FOR THE PROPIOLIC ACID – FORMIC ACID DIMER C. O. M. SEPARATION > WELL DETERMINED, BUT, RELATIVE ORIENTATIONS OF THE MONOMERS, NOT WELL DETERMINED. • TUNNELING FREQUENCY FOR HCCCOOD···DOOCH BASED ON FEW MEASUREMENTS. • HOW TO FIX THIS? • MEASURE MORE ISOTOPOLOGUES! (BOB KUCZKOWSKI RECOMMENDATION) • • • NEW MEASUREMENTS DCCCOOH···HOOCH and HCCCOOD···DOOCH isotopologues, measured in the 4. 9 -15. 4 GHz range IMPROVED STRUCTURE and OD···DO TUNNELING FREQUENCY OSU – June – 2013 - SGK 4

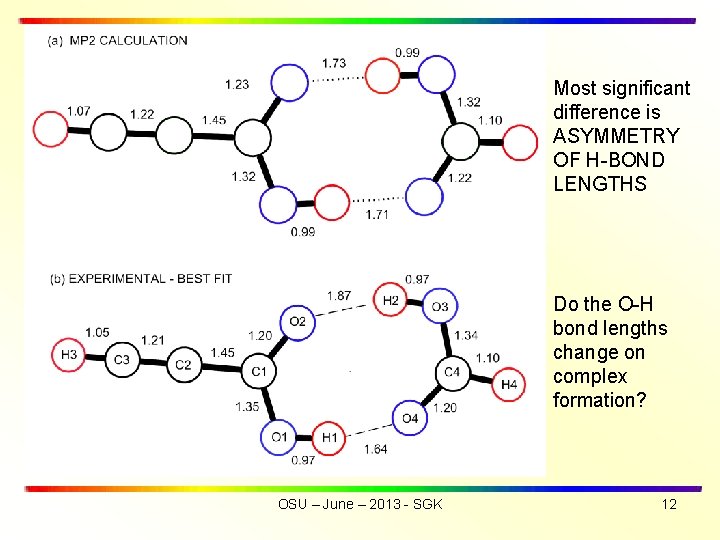
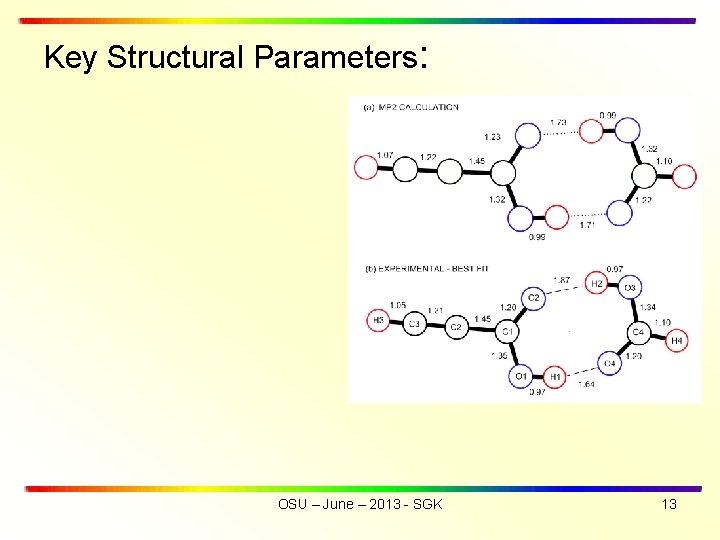
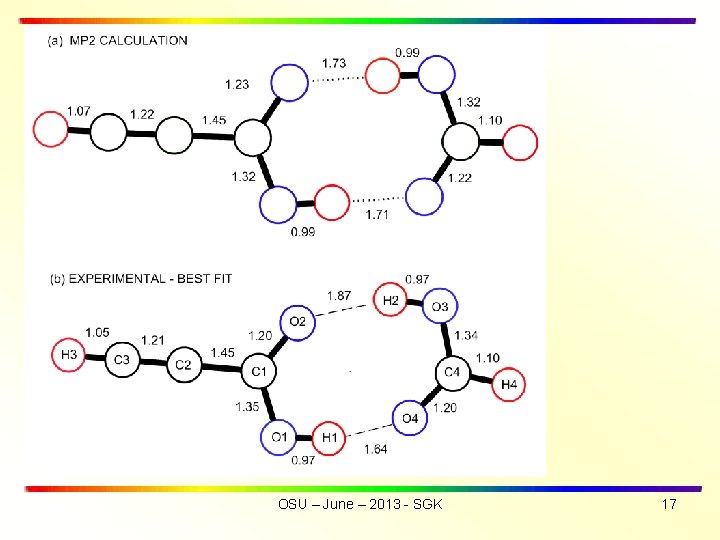
• SUMMARY OF RESULTS • Hydrogen bond lengths are r(O 1 -H 1··O 4) of 1. 64 Å and r(O 3 -H 2··O 2) 1. 87 Å. Average of hydrogen bond lengths is rav(exp) = 1. 76 Å, in good agreement with rav(theory) = 1. 72 Å. • The experimental structure exhibits a greater asymmetry for the two hydrogen bond lengths than was obtained from the ab initio mp 2 calculations • Tunneling frequency for HCCCOOD···DOOCH is = 3. 48 MHz (compared with = 291. 4 MHz for OH ···HO) OSU – June – 2013 - SGK 5

• Experimental • A new coaxial-beam FTMW spectrometer with multiple FID data acquisition completed by Ming Sun provided good resolution and sensitivity. • Propiolic acid-CD (DCCCOOH) was prepared in 32 -54% yield by the decarboxylation of acetylenedicarboxylic acid monopotassium salt in D 2 O, by Spencer Carey • 7 a-dipole transitions measured for DCCCOOH···HOOCH (lower tunneling state only) • A = 5994. , B =890. 536, C = 775. 780 MHz • Propiolic acid-OD samples were prepared using both simple hydrogen exchange with methanol(OD) (99. 5%), and by making sodium propiolate using Na. OH in methanol, and adding D 2 SO 4. • 45 transitions measured for HCCCOOD···DOOCH (a and b-dipole) OSU – June – 2013 - SGK 6

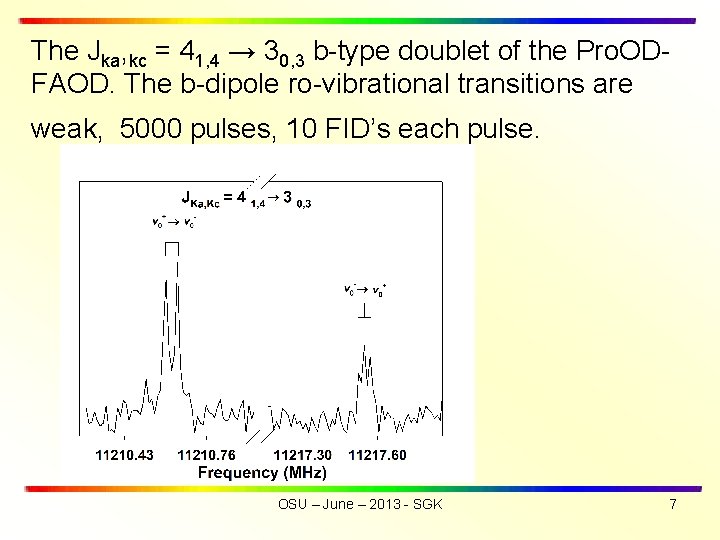
The Jka, kc = 41, 4 → 30, 3 b-type doublet of the Pro. ODFAOD. The b-dipole ro-vibrational transitions are weak, 5000 pulses, 10 FID’s each pulse. OSU – June – 2013 - SGK 7

OSU – June – 2013 - SGK 8

Determining the COM separation of the monomers is easy • The formula is: ICC = ICC(Pro) + ICC(FA) + R 2 CM. OSU – June – 2013 - SGK 9

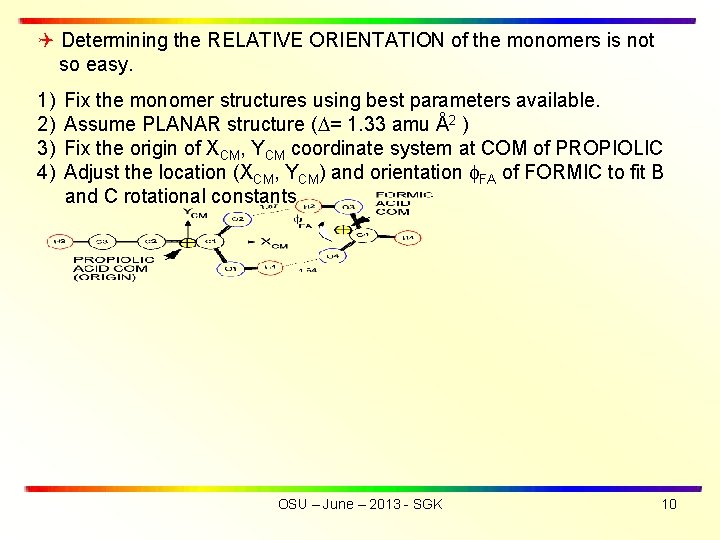
Determining the RELATIVE ORIENTATION of the monomers is not so easy. 1) Fix the monomer structures using best parameters available. 2) Assume PLANAR structure ( = 1. 33 amu Å2 ) 3) Fix the origin of XCM, YCM coordinate system at COM of PROPIOLIC 4) Adjust the location (XCM, YCM) and orientation FA of FORMIC to fit B and C rotational constants OSU – June – 2013 - SGK 10

HCOOH 1 and HCCCOOH 2 structures fixed at monomer values ISOTOPOLOGUES > HCCCOOH • • • HOOCH, HCCCOOH • • • DOOCH, HCCCOOD • • • HOOCH, HCCCOOH • • • HOO 13 CH, HCCCOOH • • • HOOCD 3, HCCCOOD • • • DOOCH, DCCCOOH • • • HOOCH (new) Experimental B and C for 7 isotopologues fit with s = 0. 7 MHz The best-fit hydrogen bond lengths are r(O 1 -H 1··O 4) = 1. 64 Å and r(O 3 -H 2··O 2) =1. 87 Å. Average (O-H) is rav(exp) = 1. 76 Å, in good agreement with rav(theory) = 1. 72 Å. Center of mass separation of monomers is RCM = 3. 864 Å. ________________ 1. Davis, R. W. ; Robiette, A. G. ; Gerry, M. C. L. ; Bjarnov, E. ; Winnewisser, G. J. Mol. Spec. 1980, 81, 93 -109. 2. Lister, D. G. ; Tyler, J. K. Spectochimica Acta 1972, 28 A, 1423 -1427. 3. Daly, A. M. ; Douglass, K. O. ; Sarkozy, L. C. ; Neill, J. L. ; Muckle, M. T. ; Zaleski, D. P. ; Pate, B. H. ; Kukolich, S. G. J. Chem. Phys. 2011, 135, 154304/1 -154304/12 OSU – June – 2013 - SGK 11

Most significant difference is ASYMMETRY OF H-BOND LENGTHS Do the O-H bond lengths change on complex formation? OSU – June – 2013 - SGK 12

Key Structural Parameters: OSU – June – 2013 - SGK 13

Acknowledgements Adam Daly (UA – JPL); Yimin Wang and Joel Bowman (Emory U. ) • Department of Chemistry, University of Arizona. • Earlier work > Phil Bunker – NRC, > Kevin Douglas, Brooks Pate – U. Virginia • N$F - This material is based upon work supported by the National Science Foundation under Grant Nos. CHE 0809053, CHE-0721505 and CHE-10557796. This support from the National Science Foundation is gratefully acknowledged OSU – June – 2013 - SGK 14

OSU – June – 2013 - SGK 15

ISOTOPOLOGUES > HCCCOOH • • • HOOCH, HCCCOOH • • • DOOCH, HCCCOOD • • • HOOCH, HCCCOOH • • • HOO 13 CH, HCCCOOH • • • HOOCD†, HCCCOOD • • • DOOCH, DCCCOOH • • • HOOCH (new) • The best-fit hydrogen bond lengths are r(O 1 -H 1··O 4) = 1. 64 Å and r(O 3 -H 2··O 2) =1. 87 Å. Average is rav(exp) = 1. 76 Å, in good agreement with rav(theory) = 1. 72 Å. Center of mass separation of monomers is RCM = 3. 864 Å. † Daly, A. M. ; Douglass, K. O. ; Sarkozy, L. C. ; Neill, J. L. ; Muckle, M. T. ; Zaleski, D. P. ; Pate, B. H. ; Kukolich, S. G. J. Chem. Phys. 2011, 135, 154304/1 -154304/12 OSU – June – 2013 - SGK 16

OSU – June – 2013 - SGK 17