METO 637 Lesson 20 Planetary Atmospheres The existence













- Slides: 20

METO 637 Lesson 20



Planetary Atmospheres • The existence of an atmosphere depends on three factors: (1) How close the planet is to the sun – basically the closer to the planet the higher the amount of heating (2) The mass of the planet. The higher the mass the larger the escape velocity has to be. (3) The strength of the magnetic field. The solar wind scows the surface of the planet unless it is diverted by a strong magnetic field, e. g. consider the Earth.


Planetary Atmospheres • Consider the planet Mercury • Its mass is only 5. 5% of the earth. • Its distance from the sun is 0. 387 of that of the earth. The solar energy per unit area is increased by a factor of 6. 7 • Temperature variations are very large 90 to 700 K. • Its core is largely solid iron – small magnetic field – 1% that of the Earth.

Planet mercury


Planet Venus

Venus • Closest planet to the Sun that has an atmosphere. Atmosphere is about 100 times as massive as the Earth’s. • Principle constituent is carbon dioxide. • Planet is covered with clouds, the lower altitude of which is at the tropopause. The clouds reflect almost all of the solar radiation – chemical change below the clouds is thermal in nature • Dense clouds lead to a run-away greenhouse effect – surface temperature ~740 K. Little water. • Small magnetic field. • The stratosphere extends from the cloud tops to 110 km.


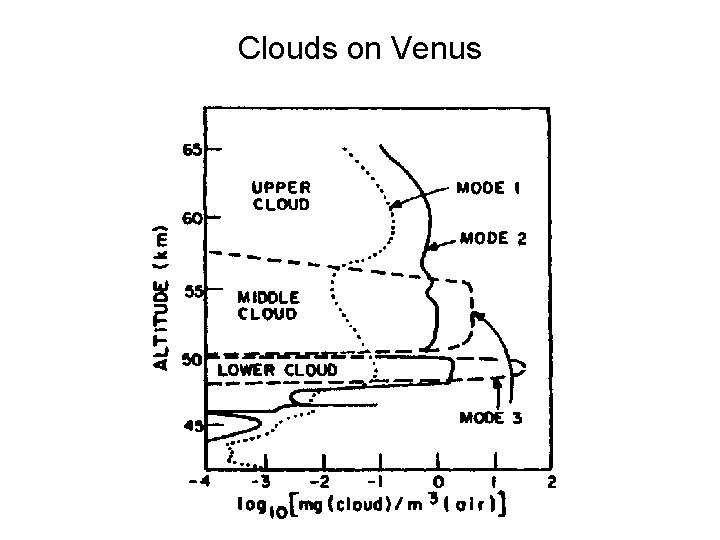
Clouds on Venus

Clouds on venus • Three major layers centered on 51, 54, and 62 km. - complex. • Three main types of cloud particles: (1) Type 1. Small aerosols – spread without the layers (2) Type 2. About one micron in size. Refractive index suggests concentrated sulfuric acid (75%) (3) Type 3. Large solid crystals found in middle and lower layers. • Other constituents within the clouds are metal chlorides, and phosphorus compounds

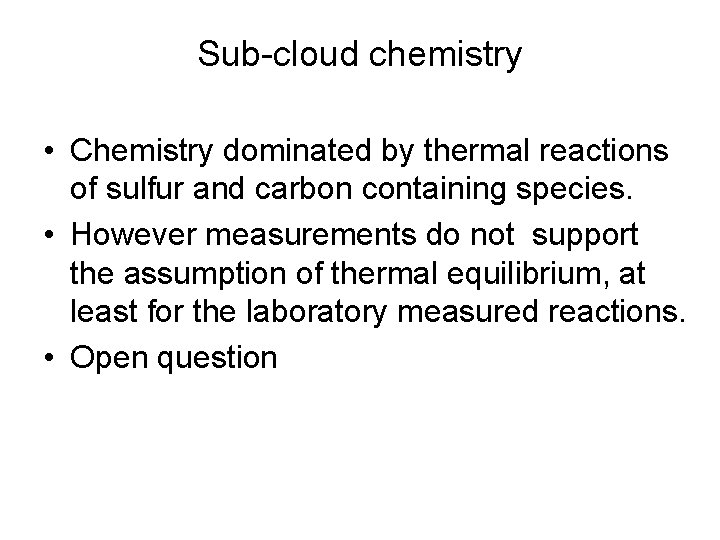
Sub-cloud chemistry • Chemistry dominated by thermal reactions of sulfur and carbon containing species. • However measurements do not support the assumption of thermal equilibrium, at least for the laboratory measured reactions. • Open question

Stratospheric chemistry • Three major questions about the chemistry of Venus: (1) What controls the extent of CO 2 photolysis – there ought to be much less CO 2 using conventional chemistry (2) What part does SO 2 play. (3) What is the abundance of H 2

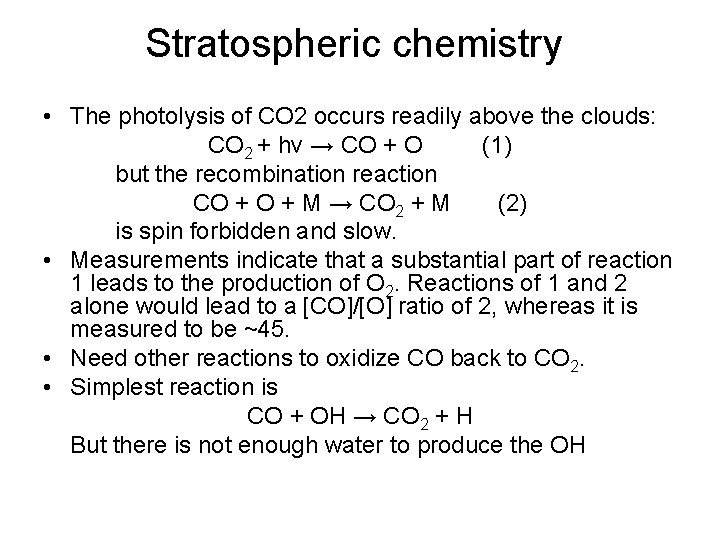
Stratospheric chemistry • The photolysis of CO 2 occurs readily above the clouds: CO 2 + hν → CO + O (1) but the recombination reaction CO + M → CO 2 + M (2) is spin forbidden and slow. • Measurements indicate that a substantial part of reaction 1 leads to the production of O 2. Reactions of 1 and 2 alone would lead to a [CO]/[O] ratio of 2, whereas it is measured to be ~45. • Need other reactions to oxidize CO back to CO 2. • Simplest reaction is CO + OH → CO 2 + H But there is not enough water to produce the OH

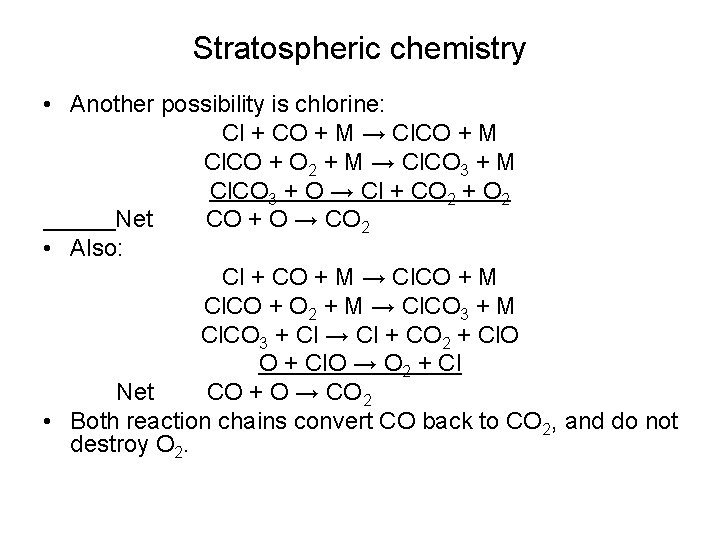
Stratospheric chemistry • Another possibility is chlorine: Cl + CO + M → Cl. CO + M Cl. CO + O 2 + M → Cl. CO 3 + M Cl. CO 3 + O → Cl + CO 2 + O 2 Net CO + O → CO 2 • Also: Cl + CO + M → Cl. CO + M Cl. CO + O 2 + M → Cl. CO 3 + M Cl. CO 3 + Cl → Cl + CO 2 + Cl. O O + Cl. O → O 2 + Cl Net CO + O → CO 2 • Both reaction chains convert CO back to CO 2, and do not destroy O 2.

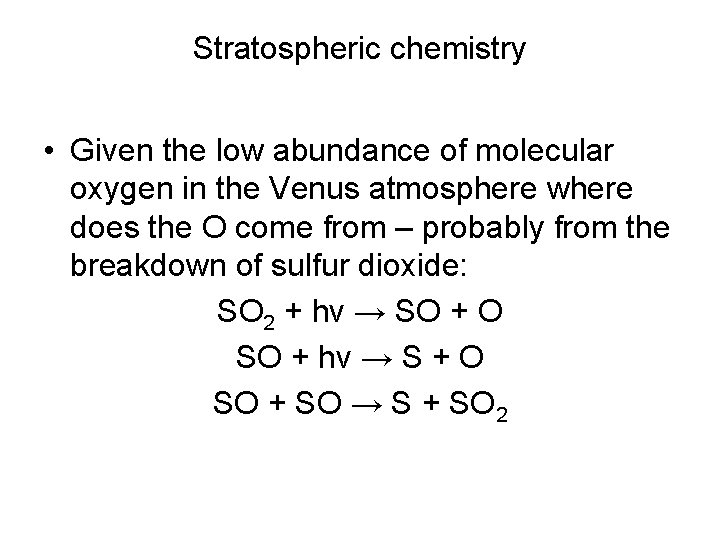
Stratospheric chemistry • Given the low abundance of molecular oxygen in the Venus atmosphere where does the O come from – probably from the breakdown of sulfur dioxide: SO 2 + hν → SO + O SO + hν → S + O SO + SO → S + SO 2


 T_gayaa
T_gayaa Lesson 1 skills practice circumference
Lesson 1 skills practice circumference Herzinsuffizienz endstadium symptome
Herzinsuffizienz endstadium symptome Como metes a un elefante en la nevera
Como metes a un elefante en la nevera Devlet meto
Devlet meto How long would it to
How long would it to Hrn hd 637 s1
Hrn hd 637 s1 Basamak adları
Basamak adları List of los angeles-class submarines
List of los angeles-class submarines Purdue medical physics
Purdue medical physics Stage 2 whole number
Stage 2 whole number Existence and uniqueness of square roots and cube roots
Existence and uniqueness of square roots and cube roots Jordan yelinek
Jordan yelinek Association of lunar and planetary observers
Association of lunar and planetary observers Prius planetary gear animation
Prius planetary gear animation Planetary temperature calculator
Planetary temperature calculator Human centered worldview
Human centered worldview Planetary systems
Planetary systems Hr 8799
Hr 8799 Planetarypositionstoday
Planetarypositionstoday 3 laws of planetary motion
3 laws of planetary motion