METO 637 Lesson 13 Air Pollution Air Pollution












- Slides: 18

METO 637 Lesson 13

Air Pollution

Air Pollution

Air Pollution

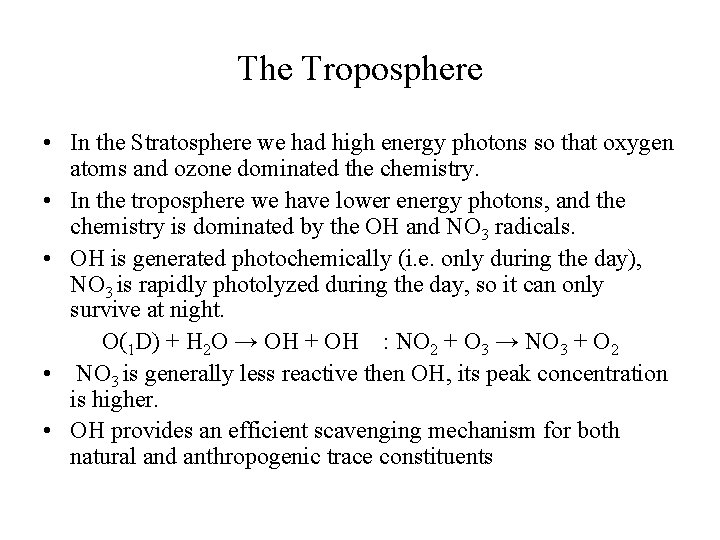
The Troposphere • In the Stratosphere we had high energy photons so that oxygen atoms and ozone dominated the chemistry. • In the troposphere we have lower energy photons, and the chemistry is dominated by the OH and NO 3 radicals. • OH is generated photochemically (i. e. only during the day), NO 3 is rapidly photolyzed during the day, so it can only survive at night. O(1 D) + H 2 O → OH + OH : NO 2 + O 3 → NO 3 + O 2 • NO 3 is generally less reactive then OH, its peak concentration is higher. • OH provides an efficient scavenging mechanism for both natural and anthropogenic trace constituents




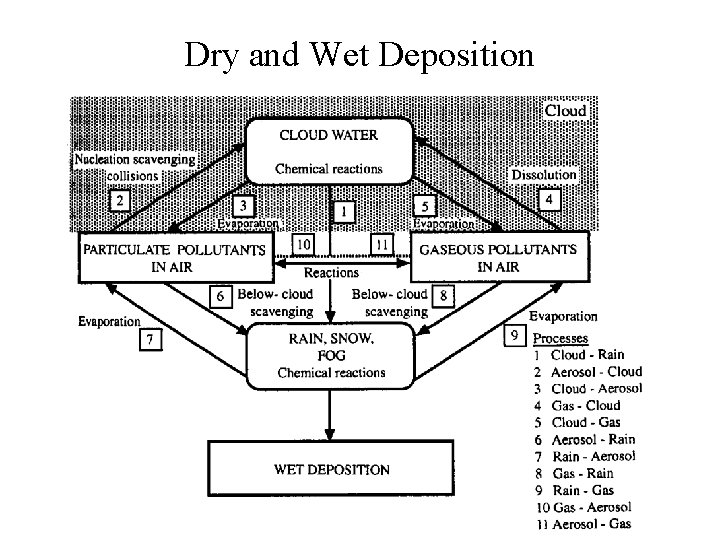
Dry and Wet Deposition • Dry deposition – removal of gases and particles by a direct transfer from the atmosphere to the surface. • Wet deposition – removal of gases and particles carried to the surface in water – rain, snow, fog etc. • Dry deposition is known for SO 2, O 3, CO 2, and SO 3. • Wet deposition of gaseous species requires that they be water soluble. Terms used are rainout, or washout. • Acid rain is an example of the rainout of sulfurous and nitric acids, produced in polluted atmospheres.

Dry Deposition • Three separate steps (1) Species must be transported close to the surface (2) Species must cross to the surface (3) Species must be taken up on the surface. • Dampness of the surface can be critical for some species, e. g. SO 2, O 3, HNO 3

Wet deposition • Wet deposition – incorporation into falling precipitation (washout), or into cloud droplets (rainout). • Chemical conversions can occur within the droplets.

Dry and Wet Deposition

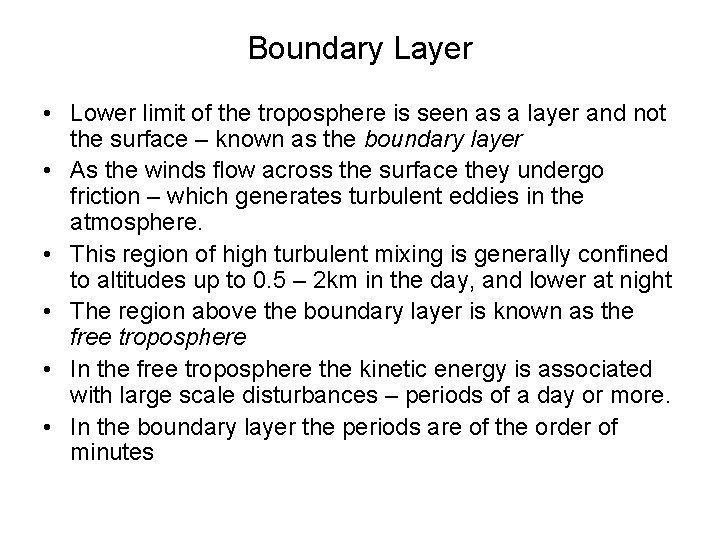
Boundary Layer • Lower limit of the troposphere is seen as a layer and not the surface – known as the boundary layer • As the winds flow across the surface they undergo friction – which generates turbulent eddies in the atmosphere. • This region of high turbulent mixing is generally confined to altitudes up to 0. 5 – 2 km in the day, and lower at night • The region above the boundary layer is known as the free troposphere • In the free troposphere the kinetic energy is associated with large scale disturbances – periods of a day or more. • In the boundary layer the periods are of the order of minutes

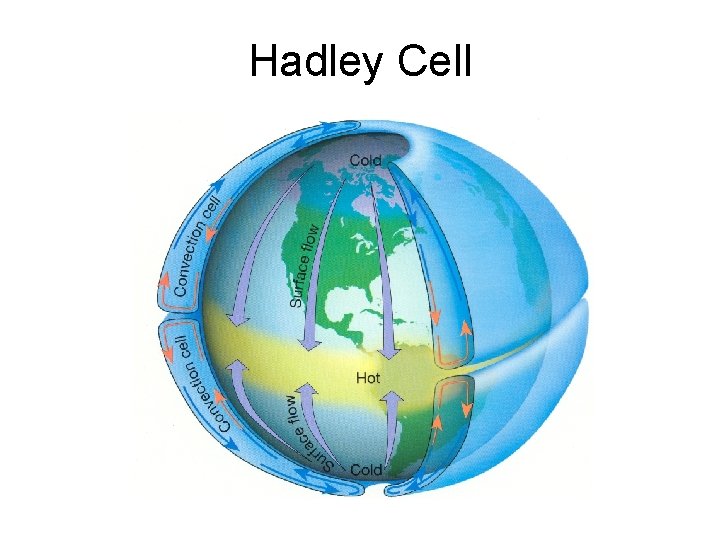
Hadley Cell

GLOBAL CIRCULATION • George Hadley first suggested in 1735 the general concept of atmospheric circulation. • He used a single cell, to explain the existence of the easterly winds at the surface • Cold air at the pole - high pressure at the surface. Warm air at the equator - low pressure at the surface. Pressure gradient force at surface will move air from pole to equator at surface. Return path at high altitudes. • The action of the Coriolis force on this southerly flow at the surface will produce easterly winds. • But we find westerly winds at mid-latitudes – why?

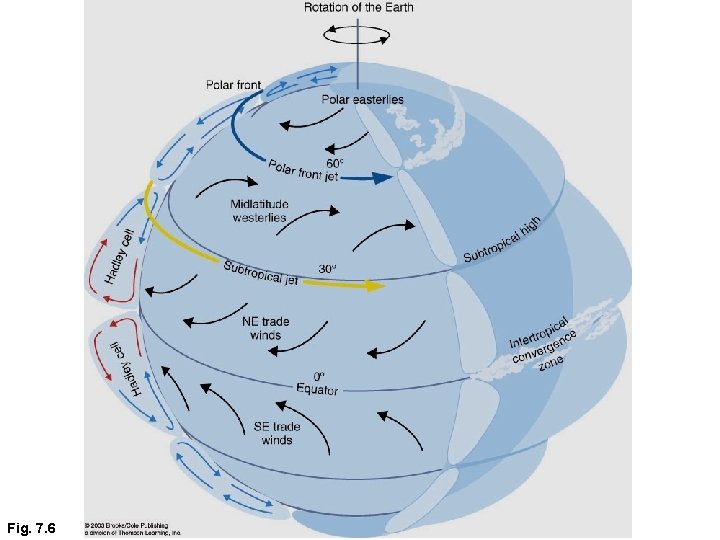
GLOBAL CIRCULATION • In reality the atmosphere cannot maintain a single cell, instead there are three cells, with boundaries at about 30 and 60 degrees latitude. • This results in sinking air at 30 N and 30 S. But sinking air suppresses cloud development and precipitation. Hence most of the worlds deserts occur along these latitudes. • At mid-latitudes the prevailing wind in the free troposphere is westerly (mid-latitude westerlies) • Between the equator and 30 degrees we get easterlies – trade winds. • The free troposphere also has lower temperatures than at the surface – reaction rates are slower, and pollutants can travel large distances before they are removed chemically.

Fig. 7. 6

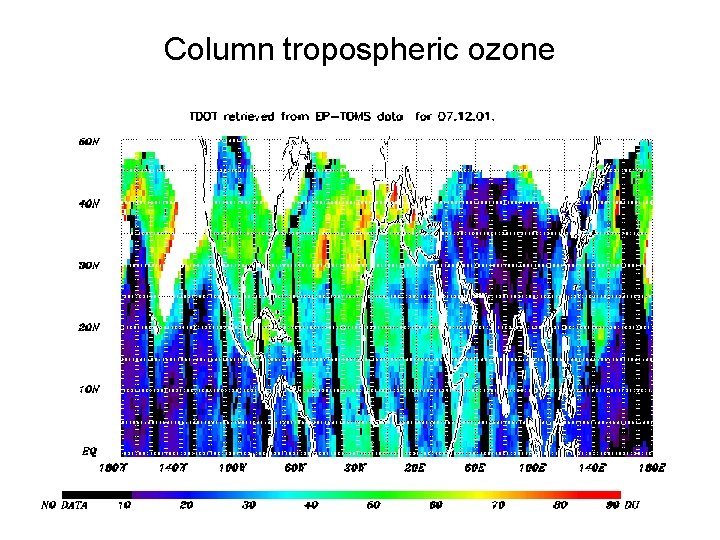
Column tropospheric ozone
 T_gayaa
T_gayaa Lesson 2 reteach area of circles answer key
Lesson 2 reteach area of circles answer key Meto zerok 25 mg nebenwirkungen
Meto zerok 25 mg nebenwirkungen Como meter un elefante en una nevera
Como meter un elefante en una nevera Devlet meto
Devlet meto Peta scientific notation
Peta scientific notation Hrn hd 637 s1
Hrn hd 637 s1 Basamak adları
Basamak adları List of los angeles-class submarines
List of los angeles-class submarines Purdue medical physics
Purdue medical physics Place value learning intentions
Place value learning intentions Chapter 12 section 3 acid precipitation
Chapter 12 section 3 acid precipitation Chapter 12 air section 1 what causes air pollution
Chapter 12 air section 1 what causes air pollution Hubungan air tanah dan tanaman
Hubungan air tanah dan tanaman Soil pollution effects on human health
Soil pollution effects on human health Measures of noise pollution
Measures of noise pollution Soil pollution images diagram
Soil pollution images diagram Introduction of pollution
Introduction of pollution Effects of air pollution to plants
Effects of air pollution to plants