Mepact mifamurtide Fulfilling an unmet need in osteosarcoma




- Slides: 42

Mepact® (mifamurtide) Fulfilling an unmet need in osteosarcoma treatment Prescribing information can be found at end of the presentation EUCAN/MEP/2013 -10019 September 2013

Osteosarcoma ● Second most common type of primary bone cancer 1 ● Disease of children and young adults 2 – Most common between ages of 10 -20 years 2 – Accounts for >10% of all solid tumours in adolescents aged 15 -19 years 3 ● More common in males than females 3 ● Incidence: 0. 2 -0. 3 per 100, 000 population in Europe 3 – Estimated 1, 135 new cases every year in Europe 1 1. Stiller CA et al. Eur J Cancer 2013; 49: 684 -695. 2. Meyers PA, Gorlick R. Pediatr Clin North Am 1997; 44(4): 973 -990. 3. Bone sarcomas: ESMO Clinical Practice Guidelines. Ann Oncol 2012; 23(7): vii 100 -vii 109. EUCAN/MEP/2013 -10019 September 2013

Osteosarcoma ● Most common sites are ends of long bones 1 – Approximately half of tumours begin around the knee ● Around 20% of newly diagnosed patients have detectable lung metastases 1 – Almost all patients have subclinical microscopic metastases – Death from osteosarcoma is almost always the result of progressive pulmonary metastases ● Relapse rate in newly diagnosed patients can be as high as 30% 2 – Most recurrences occur as lung metastases 1 1. Meyers PA, Gorlick R. Pediatr Clin North Am 1997; 44(4): 973 -990. 2. Grimer RJ. Lancet Oncol 2005: 6: 85 -92. EUCAN/MEP/2013 -10019 September 2013

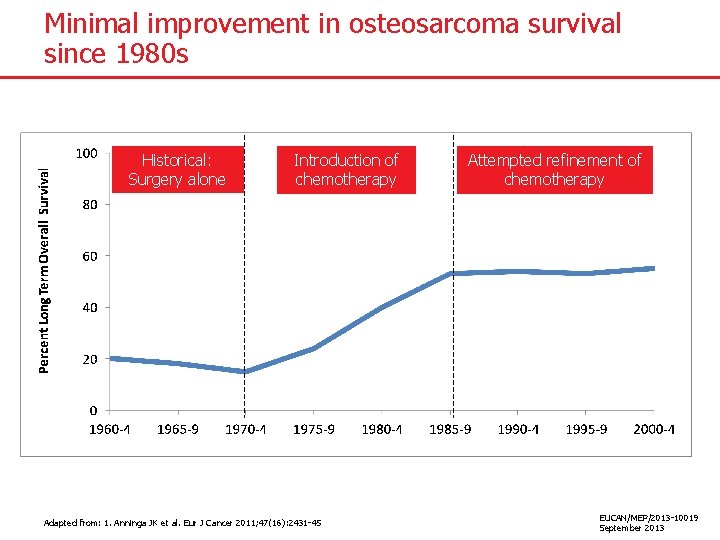
Minimal improvement in osteosarcoma survival since 1980 s Historical: Surgery alone Introduction of chemotherapy Adapted from: 1. Anninga JK et al. Eur J Cancer 2011; 47(16): 2431 -45 Attempted refinement of chemotherapy EUCAN/MEP/2013 -10019 September 2013

Minimal improvement in osteosarcoma survival since 1980 s ● Before the 1970 s patients underwent amputation and no chemotherapy – Long term overall survival was <20%1 ● By the mid 1980 s long term overall survival up to 50 -60% 1 ● Some studies showed better rates, but overall little significant improvement for over three decades 1 ● Meta-analysis of chemotherapy studies showed 1: – 5 year overall survival of 60% with 2 -drug regimen compared with 66% for 3 -drug regimen (p=0. 04) – No benefit of a fourth drug ● Most investigators agree treatment has reached a plateau 2 1. Anninga JK et al. Eur J Cancer 2011; 47(16): 2431 -45. 2. Meyers PA. Expert Revs Anticancer Ther 2009; 9: 1035 -1049. EUCAN/MEP/2013 -10019 September 2013

Significant unmet need in osteosarcoma treatment ● Today, long-term (5 -year) survival for localised disease is 50 -60% 1 ● Continuing improvements in survival have been seen for many childhood cancers, but the news is less positive for osteosarcoma 2 − No improvement in past 30 years despite the introduction of multiagent chemotherapy 1 ● Therefore, improving overall survival for osteosarcoma patients represents a significant unmet need 1. Anninga JK et al. Eur J Cancer 2011; 47(16): 2431 -45 2. Gatta G et al. J Clin Oncol 2005; 23: 3742 -3751 EUCAN/MEP/2013 -10019 September 2013

The potential role of immunotherapy in osteosarcoma ● Immunotherapy is a treatment strategy which upregulates the body’s immune response to cancer cells and thus increases tumouricidal activity 1 ● There is a growing body of evidence to support immunotherapy as an effective treatment in osteosarcoma 1 ● Postoperative infection after endoprosthetic surgery has been shown to improve survival outcome which suggested a role for infectioninduced anti-tumour immune response 2 1. Mori K et al. Oncology Reports 2006; 15: 693 -700 2. Jeys LM et al. Ann Surg Oncol. 2007; 14(10): 2887 -95 EUCAN/MEP/2013 -10019 September 2013

The evidence for immunotherapy in osteosarcoma ● Potential strategies targeting the host immune system include: - Immune modulation (interferon-α 1, muramyl tripeptide phosphatidyl ethanolamine MTP-PE 2) - Cellular therapy (cytotoxic T-cells, primed dendritic cells 3) ● Study results cannot be extrapolated across immunotherapeutic agents: – Immunotherapy with interferon-α as an adjuvant therapy has been evaluated following initial encouraging results 1 however preliminary data from the EURAMOS-1 trial concluded that there is no evidence to support interferon-α in this setting 4 – Mepact has demonstrated a proven benefit on overall survival 2 1. 2. 3. 4. Müller C et al. Acta Oncologica 2005; 44: 475 -480 Meyers PA et al. J Clin Oncol 2008; 26(4): 633 -638 Mori K et al. Oncology Reports 2006; 15: 693 -700 Bielack S et al. J Clin Oncol 31, 2013 (suppl; abstr LBA 10504) EUCAN/MEP/2013 -10019 September 2013

Introduction - What is Mepact? ● Mepact (mifamurtide) is L-MTP-PE, a liposomal formulation of Muramyl Tripeptide Phosphatidyl Ethanolamine (MTP-PE)1 – Liposomal formulation facilitates uptake by tissue macrophages after i. v. infusion – A potent activator of macrophages ● L-MTP-PE was initially developed as an immunostimulant with significant anti-tumour effects in preclinical models 1, 2 ● MTP-PE is a synthetic analog of muramyl dipeptide (MDP)3 – The smallest natural immune stimulatory component of the mycobacterium cell wall 1 1. Nardin A. et al. Current Cancer Drug Targets. 2006; 6: 123 -133 2. Fidler IJ. et al. Proc. Natl Acad. Sci. USA. 1981; 78: 1680 -1684 3. Mepact Sm. PC, March 2013 http: //www. medicines. org. uk/emc/medicine/22763 EUCAN/MEP/2013 -10019 September 2013

Mepact is well studied and characterised ● Extensive supporting data at the time of Marketing Authorisation submission (November 2006) – Administered to more than 700 patients in clinical trials 1 – More than 100 pre-clinical studies 1 ● 86 pharmacology studies, 12 pharmacokinetic studies and 20 toxicology studies were submitted as part of the EMA dossier 1 – Extensive clinical development program ● 18 Phase I and II studies were submitted as part of EMA dossier 1 1. EMA Assessment Report for Mepact EMEA/H/C/000802. EUCAN/MEP/2013 -10019 September 2013

Mepact is well studied and characterised ● Robust Phase III clinical data 1 – Leading paediatric cooperative study group (COG*) clinical trial – One of the largest studies ever completed – Consistent, significant and long-term survival benefit ● Almost one third reduction in relative risk of death with an absolute risk reduction of 8% (p=0. 03)1 – Favourable benefit/risk profile 2 ● Granted Orphan Medicinal Product Status in 20043 ● One of most studied paediatric oncology drugs * COG – Children’s Oncology Group 1. Meyers PA et al. J Clin Oncol. 2008; 26: 633 -638. 2. Meyers PA. Expert Revs Anticancer Ther 2009; 9: 1035 -1049. 3. EMA. EMEA/COMP/373/04 EUCAN/MEP/2013 -10019 September 2013

Mepact development pathway ● In pre-clinical models, Mepact was proven to be an immunostimulant with significant anti-tumour effects 1 – Anti-tumour effects are macrophage mediated ● Direct (activated macrophage tumour killing) ● Indirect (cytokine mediated, activation of immune cells) ● Liposomal formulation facilitates uptake by tissue macrophages after i. v. injection 1 – Particularly in lungs, nasopharynx, spleen, liver 2 ● Following pre-clinical models, results in dogs stimulated additional interest in Mepact 3 – Comparable model for human osteosarcoma 1. Nardin A et al. Current Cancer Drug Targets. 2006; 6: 123 -133. 2. Murray JL et al. J Clin Oncol. 1989; 7: 1915 -1925. 3. Meyers PA. Expert Revs Anticancer Ther 2009; 9: 1035 -1049. EUCAN/MEP/2013 -10019 September 2013

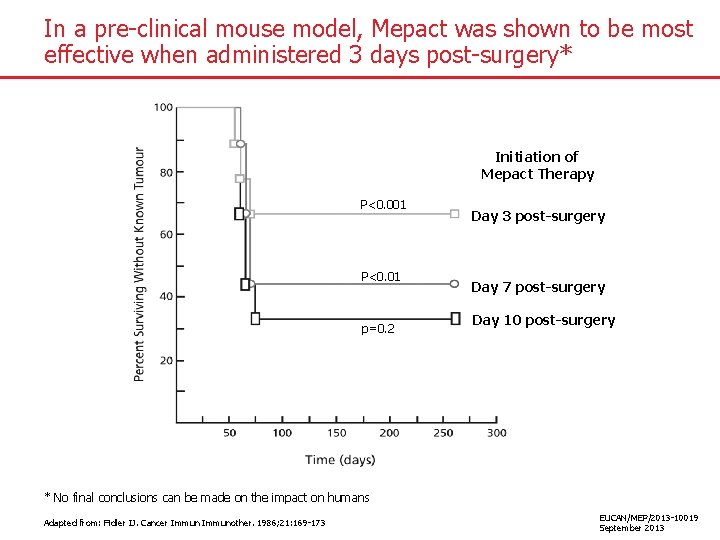
In a pre-clinical mouse model, Mepact was shown to be most effective when administered 3 days post-surgery* Initiation of Mepact Therapy P<0. 001 P<0. 01 p=0. 2 Day 3 post-surgery Day 7 post-surgery Day 10 10 post-surgery Day * No final conclusions can be made on the impact on humans Adapted from: Fidler IJ. Cancer Immunother. 1986; 21: 169 -173 EUCAN/MEP/2013 -10019 September 2013

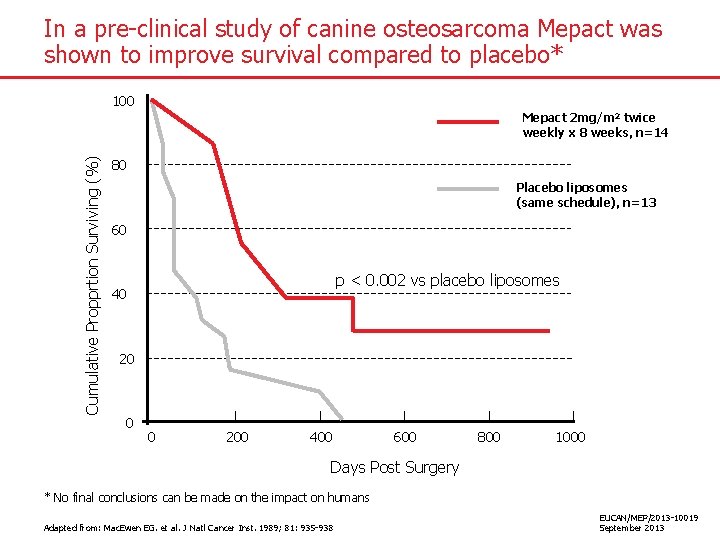
In a pre-clinical study of canine osteosarcoma Mepact was shown to improve survival compared to placebo* 100 Cumulative Propprtion Surviving (%) Mepact 2 mg/m 2 twice weekly x 8 weeks, n=14 80 Placebo liposomes (same schedule), n=13 60 p < 0. 002 vs placebo liposomes 40 20 0 0 200 400 600 800 1000 Days Post Surgery * No final conclusions can be made on the impact on humans Adapted from: Mac. Ewen EG. et al. J Natl Cancer Inst. 1989; 81: 935 -938 EUCAN/MEP/2013 -10019 September 2013

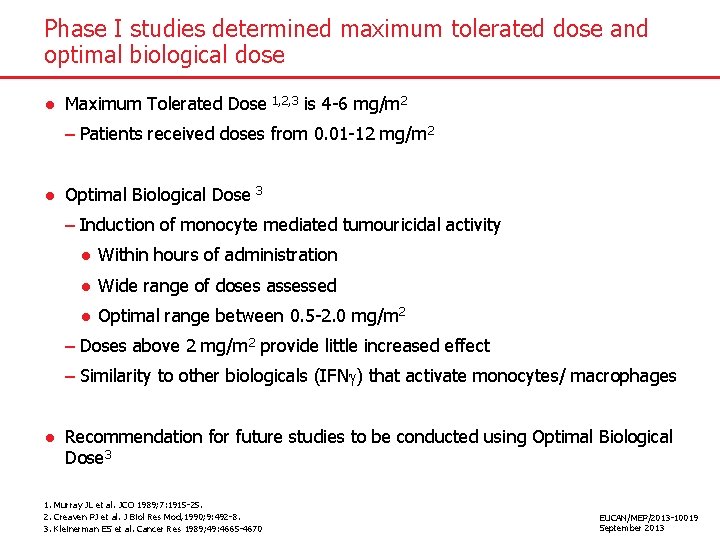
Phase I studies determined maximum tolerated dose and optimal biological dose ● Maximum Tolerated Dose 1, 2, 3 is 4 -6 mg/m 2 – Patients received doses from 0. 01 -12 mg/m 2 ● Optimal Biological Dose 3 – Induction of monocyte mediated tumouricidal activity ● Within hours of administration ● Wide range of doses assessed ● Optimal range between 0. 5 -2. 0 mg/m 2 – Doses above 2 mg/m 2 provide little increased effect – Similarity to other biologicals (IFN ) that activate monocytes/ macrophages ● Recommendation for future studies to be conducted using Optimal Biological Dose 3 1. Murray JL et al. JCO 1989; 7: 1915 -25. 2. Creaven PJ et al. J Biol Res Mod, 1990; 9: 492 -8. 3. Kleinerman ES et al. Cancer Res 1989; 49: 4665 -4670 EUCAN/MEP/2013 -10019 September 2013

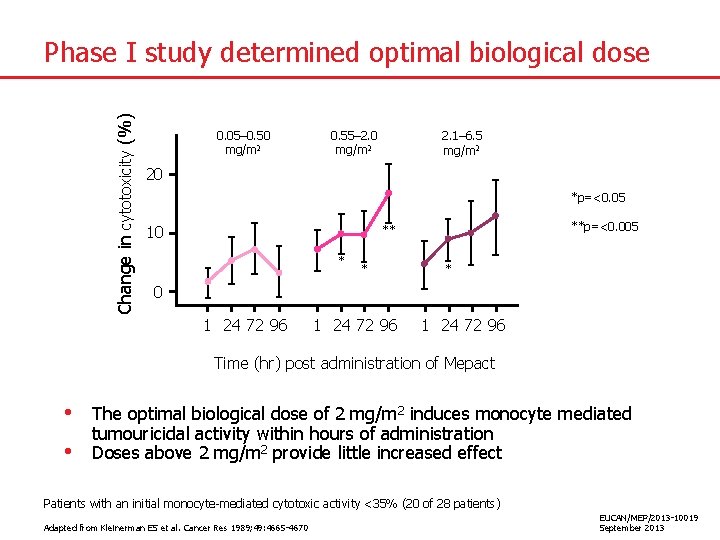
Change in cytotoxicity (%) Phase I study determined optimal biological dose 0. 05– 0. 50 mg/m 2 0. 55– 2. 0 mg/m 2 2. 1– 6. 5 mg/m 2 20 *p=<0. 05 10 **p=<0. 005 ** * 0 1 24 72 96 Time (hr) post administration of Mepact • • The optimal biological dose of 2 mg/m 2 induces monocyte mediated tumouricidal activity within hours of administration Doses above 2 mg/m 2 provide little increased effect Patients with an initial monocyte-mediated cytotoxic activity <35% (20 of 28 patients) Adapted from Kleinerman ES et al. Cancer Res 1989; 49: 4665 -4670 EUCAN/MEP/2013 -10019 September 2013

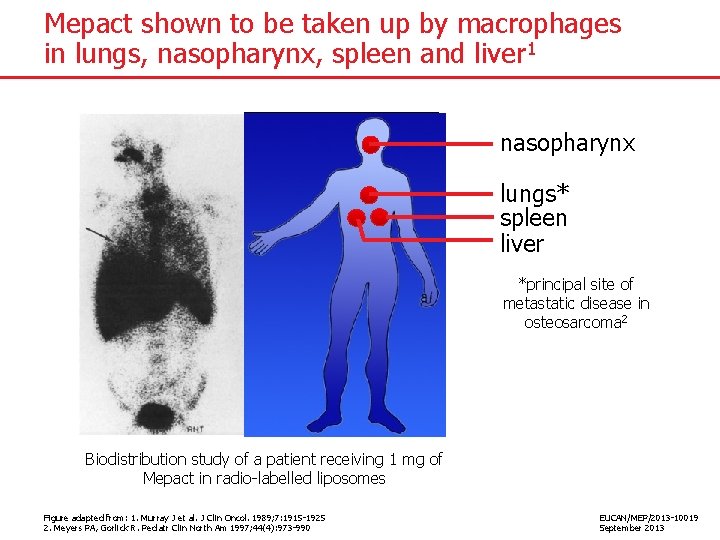
Mepact shown to be taken up by macrophages in lungs, nasopharynx, spleen and liver 1 nasopharynx lungs* spleen liver *principal site of metastatic disease in osteosarcoma 2 Biodistribution study of a patient receiving 1 mg of Mepact in radio-labelled liposomes Figure adapted from: 1. Murray J et al. J Clin Oncol. 1989; 7: 1915 -1925 2. Meyers PA, Gorlick R. Pediatr Clin North Am 1997; 44(4): 973 -990 EUCAN/MEP/2013 -10019 September 2013

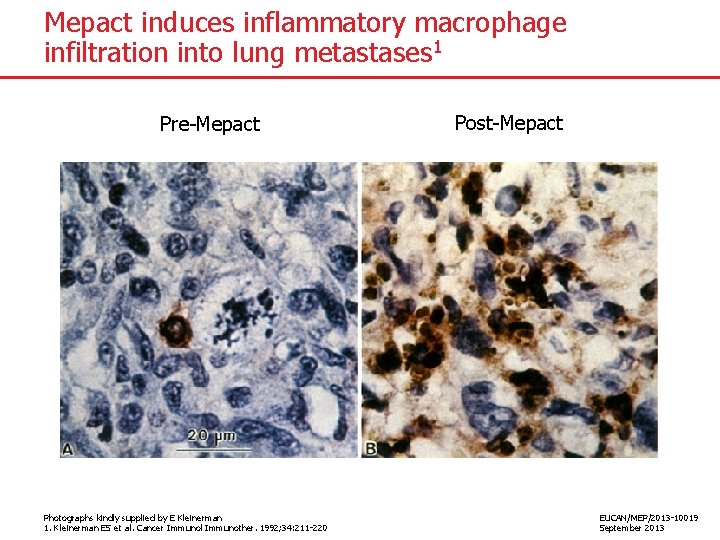
Mepact induces inflammatory macrophage infiltration into lung metastases 1 Pre-Mepact Photographs kindly supplied by E Kleinerman 1. Kleinerman ES et al. Cancer Immunol Immunother. 1992; 34: 211 -220 Post-Mepact EUCAN/MEP/2013 -10019 September 2013

Measured immunological effects ● Activation of macrophages 1 ● Induction of tumouricidal monocytes 2 ● Elevated levels: – IL-12, 3 −IL-24 – IL-61, 3 -7 −IL-83, 7 – IL-123 −TNF- 1, 3, 4, 7 – C-reactive protein (CRP)1, 4, 7 −Neopterin 4, 6 -8 ● Increased levels of β 2 -microglobulin 6 ● Leukocytosis 8 1. 2. 3. 4. 5. 6. 7. 8. Kleinerman ES et al. J Clin Onco. 1992; 10: 1310 -1316. Kleinerman ES et al. Cancer Research 1989; 49: 4665 -4670. Mepact Sm. PC, March 2013 http: //www. medicines. org. uk/emc/medicine/22763 Sculier JP et al. Drug Invest. 1993; 6: 276 -284. Frost H et al. J Biol Response Modifiers. 1990; 9: 160 -166. Liebes L et al. J Natl Cancer Inst. 1992; 84: 694 -699. Asano T. Kleinerman ES. J Immunother. 1993; 14: 286 -292. Landmann R et al. Biotherapy. 1994; 7: 1 -12. EUCAN/MEP/2013 -10019 September 2013

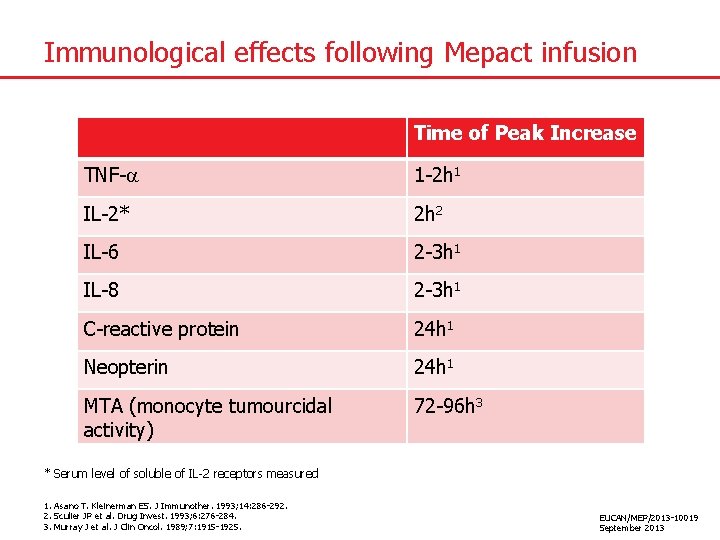
Immunological effects following Mepact infusion Time of Peak Increase TNF- 1 -2 h 1 IL-2* 2 h 2 IL-6 2 -3 h 1 IL-8 2 -3 h 1 C-reactive protein 24 h 1 Neopterin 24 h 1 MTA (monocyte tumourcidal activity) 72 -96 h 3 * Serum level of soluble of IL-2 receptors measured 1. Asano T. Kleinerman ES. J Immunother. 1993; 14: 286 -292. 2. Sculier JP et al. Drug Invest. 1993; 6: 276 -284. 3. Murray J et al. J Clin Oncol. 1989; 7: 1915 -1925. EUCAN/MEP/2013 -10019 September 2013

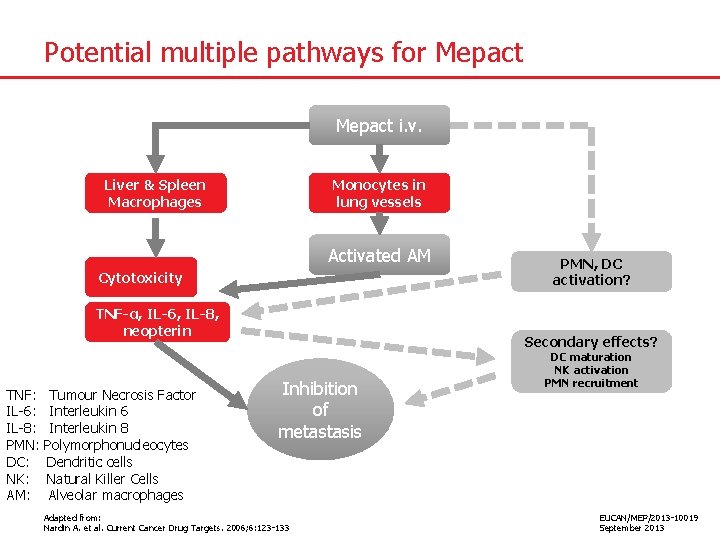
Potential multiple pathways for Mepact i. v. Liver & Spleen Macrophages Monocytes in lung vessels Activated AM Cytotoxicity TNF-α, IL-6, IL-8, neopterin TNF: IL-6: IL-8: PMN: DC: NK: AM: Tumour Necrosis Factor Interleukin 6 Interleukin 8 Polymorphonucleocytes Dendritic cells Natural Killer Cells Alveolar macrophages PMN, DC activation? Secondary effects? Inhibition of metastasis Adapted from: Nardin A. et al. Current Cancer Drug Targets. 2006; 6: 123 -133 DC maturation NK activation PMN recruitment EUCAN/MEP/2013 -10019 September 2013

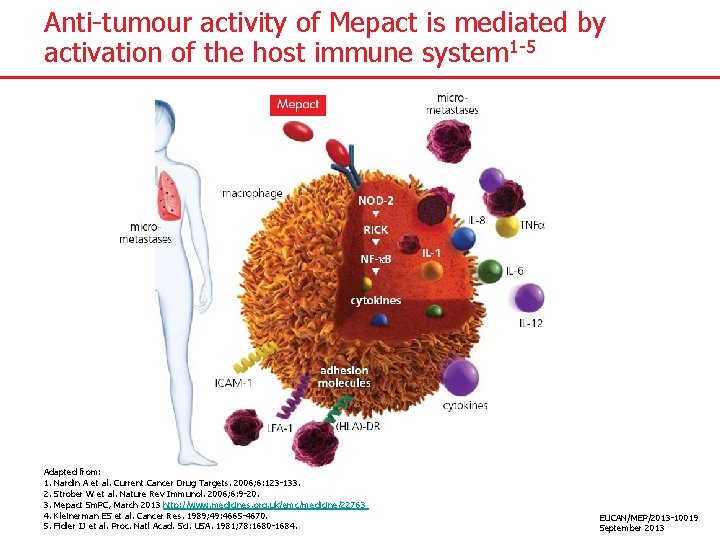
Anti-tumour activity of Mepact is mediated by activation of the host immune system 1 -5 Adapted from: 1. Nardin A et al. Current Cancer Drug Targets. 2006; 6: 123 -133. 2. Strober W et al. Nature Rev Immunol. 2006; 6: 9 -20. 3. Mepact Sm. PC, March 2013 http: //www. medicines. org. uk/emc/medicine/22763 4. Kleinerman ES et al. Cancer Res. 1989; 49: 4665 -4670. 5. Fidler IJ et al. Proc. Natl Acad. Sci. USA. 1981; 78: 1680 -1684. EUCAN/MEP/2013 -10019 September 2013

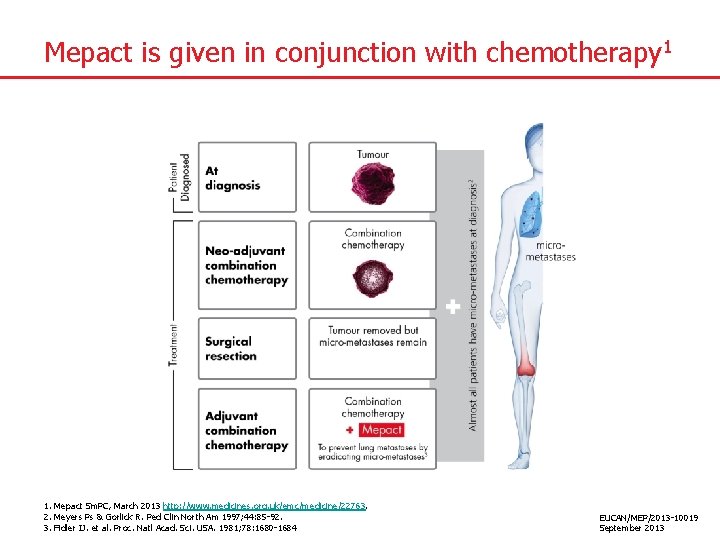
Mepact is given in conjunction with chemotherapy 1 1. Mepact Sm. PC, March 2013 http: //www. medicines. org. uk/emc/medicine/22763. 2. Meyers Ps & Gorlick R. Ped Clin North Am 1997; 44: 85 -92. 3. Fidler IJ. et al. Proc. Natl Acad. Sci. USA. 1981; 78: 1680 -1684 EUCAN/MEP/2013 -10019 September 2013

Mepact administration ● Supplied as lyophilised powder – Liposome suspensions are formed after hydration of lyophilisate ● 48 Doses over 36 weeks – 2 mg/m 2 – One hour i. v. infusion Examples only – replace with vial and pack shots appropriate to your market – First 12 weeks: ● Twice weekly ● Total 24 doses – Next 24 weeks: ● Once weekly ● Total 24 infusions Mepact Sm. PC, March 2013 http: //www. medicines. org. uk/emc/medicine/22763 EUCAN/MEP/2013 -10019 September 2013

The Children’s Oncology Group Phase III study of Mepact ● National Cancer Institute sponsored Children’s Oncology Group (COG) study 1, 2 – Designed and conducted independently of corporate sponsor – 1993 -1997 enrollment – 1993 -2007 follow-up ● One of the largest studies completed in osteosarcoma ● Newly diagnosed patients (enrolled n=793*; age <31)2 – 662 non-metastatic resectable disease – 115 with more metastatic or unresectable disease ● Independently analysed and published survival benefit 2 – Meyers PA, Schwartz CL, Krailo MK et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival - A report from the Children’s Oncology Group. J Clin Oncol 2008; 26: 633 -638 * 777 patients entered the study. 16 were ineligible for treatment 1. 2. Meyers PA. et al. J Clin Oncol. 2005; 23: 2004 -2011. Meyers PA. et al. J Clin Oncol. 2008; 26: 633 -638. EUCAN/MEP/2013 -10019 September 2013

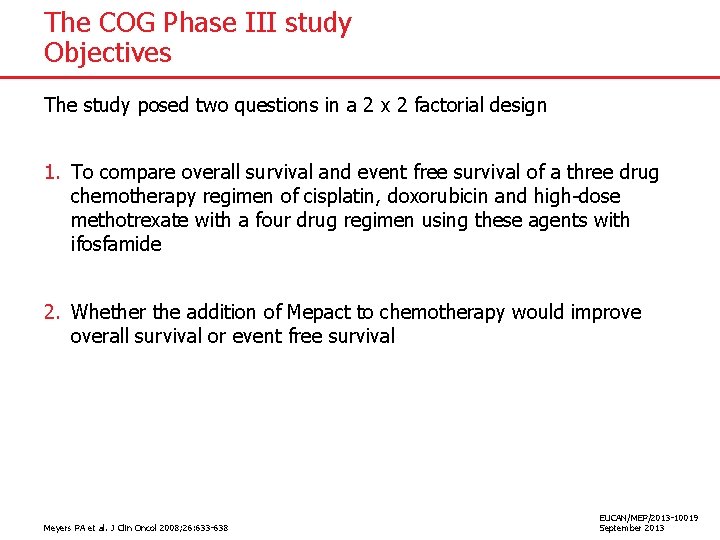
The COG Phase III study Objectives The study posed two questions in a 2 x 2 factorial design 1. To compare overall survival and event free survival of a three drug chemotherapy regimen of cisplatin, doxorubicin and high-dose methotrexate with a four drug regimen using these agents with ifosfamide 2. Whether the addition of Mepact to chemotherapy would improve overall survival or event free survival Meyers PA et al. J Clin Oncol 2008; 26: 633 -638 EUCAN/MEP/2013 -10019 September 2013

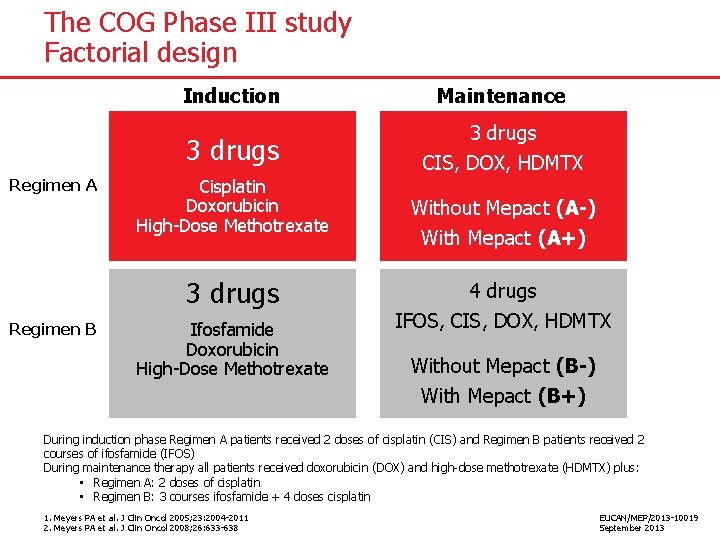
The COG Phase III study Factorial design Induction 3 drugs Regimen A Cisplatin Doxorubicin High-Dose Methotrexate 3 drugs Regimen B Ifosfamide Doxorubicin High-Dose Methotrexate Maintenance 3 drugs CIS, DOX, HDMTX Without Mepact (A-) With Mepact (A+) 4 drugs IFOS, CIS, DOX, HDMTX Without Mepact (B-) With Mepact (B+) During induction phase Regimen A patients received 2 doses of cisplatin (CIS) and Regimen B patients received 2 courses of ifosfamide (IFOS) During maintenance therapy all patients received doxorubicin (DOX) and high-dose methotrexate (HDMTX) plus: • Regimen A: 2 doses of cisplatin • Regimen B: 3 courses ifosfamide + 4 doses cisplatin 1. Meyers PA et al. J Clin Oncol 2005; 23: 2004 -2011 2. Meyers PA et al. J Clin Oncol 2008; 26: 633 -638 EUCAN/MEP/2013 -10019 September 2013

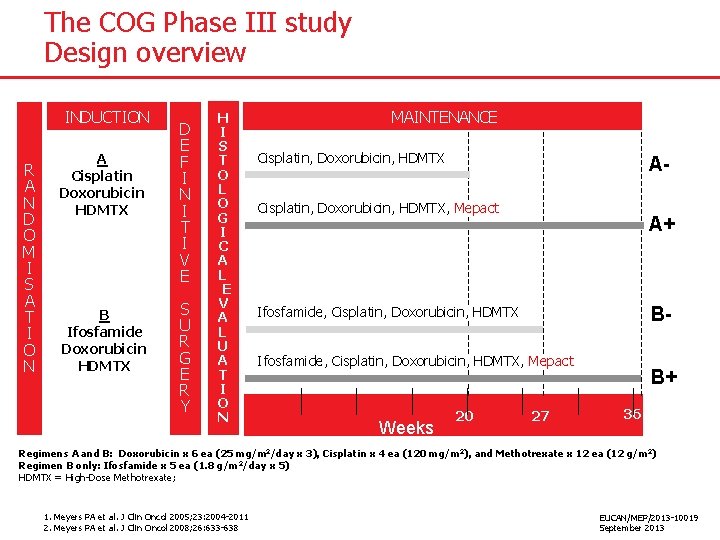
The COG Phase III study Design overview INDUCTION R A N D O M I S A T I O N A Cisplatin Doxorubicin HDMTX B Ifosfamide Doxorubicin HDMTX D E F I N I T I V E S U R G E R Y H I S T O L O G I C A L E V A L U A T I O N MAINTENANCE Cisplatin, Doxorubicin, HDMTX A- Cisplatin, Doxorubicin, HDMTX, Mepact A+ B- Ifosfamide, Cisplatin, Doxorubicin, HDMTX, Mepact Weeks 20 27 B+ 36 Regimens A and B: Doxorubicin x 6 ea (25 mg/m 2/day x 3), Cisplatin x 4 ea (120 mg/m 2), and Methotrexate x 12 ea (12 g/m 2) Regimen B only: Ifosfamide x 5 ea (1. 8 g/m 2/day x 5) HDMTX = High-Dose Methotrexate; 1. Meyers PA et al. J Clin Oncol 2005; 23: 2004 -2011 2. Meyers PA et al. J Clin Oncol 2008; 26: 633 -638 EUCAN/MEP/2013 -10019 September 2013

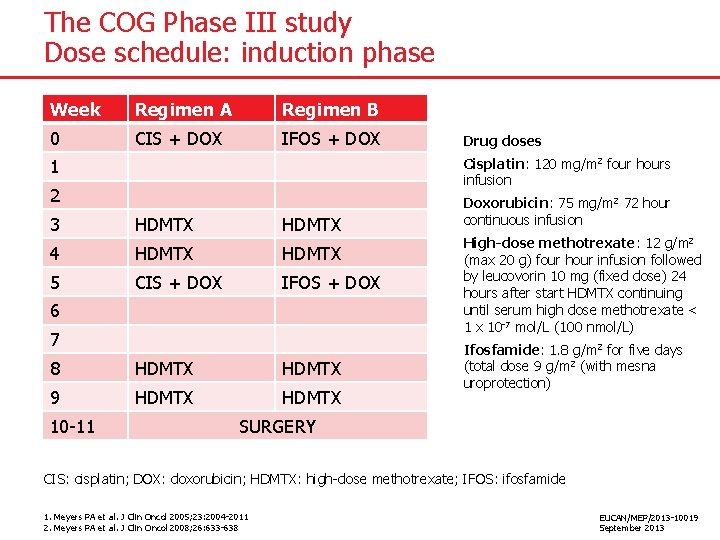
The COG Phase III study Dose schedule: induction phase Week Regimen A Regimen B 0 CIS + DOX IFOS + DOX Drug doses Cisplatin: 120 mg/m 2 four hours infusion 1 2 Doxorubicin: 75 mg/m 2 72 hour continuous infusion 3 HDMTX 4 HDMTX 5 CIS + DOX IFOS + DOX High-dose methotrexate: 12 g/m 2 (max 20 g) four hour infusion followed by leucovorin 10 mg (fixed dose) 24 hours after start HDMTX continuing until serum high dose methotrexate < 1 x 10 -7 mol/L (100 nmol/L) Ifosfamide: 1. 8 g/m 2 for five days (total dose 9 g/m 2 (with mesna uroprotection) 6 7 8 HDMTX 9 HDMTX 10 -11 SURGERY CIS: cisplatin; DOX: doxorubicin; HDMTX: high-dose methotrexate; IFOS: ifosfamide 1. Meyers PA et al. J Clin Oncol 2005; 23: 2004 -2011 2. Meyers PA et al. J Clin Oncol 2008; 26: 633 -638 EUCAN/MEP/2013 -10019 September 2013

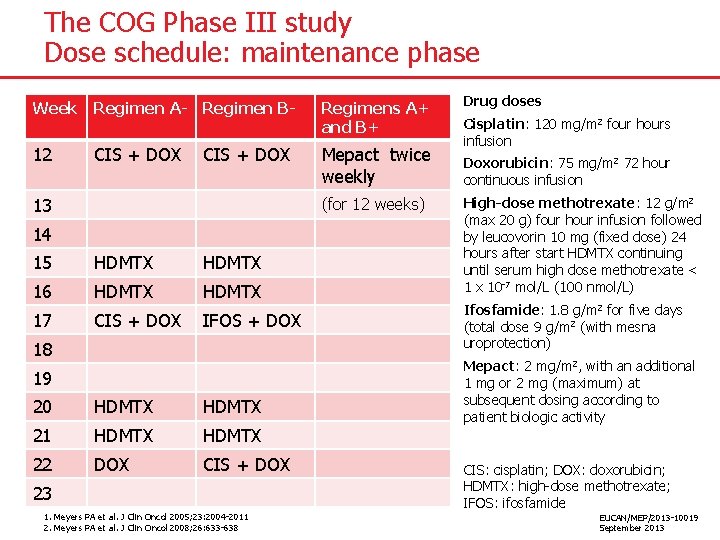
The COG Phase III study Dose schedule: maintenance phase Week Regimen A- Regimen B- Regimens A+ and B+ 12 CIS + DOX Mepact twice weekly CIS + DOX 13 (for 12 weeks) 14 15 HDMTX 16 HDMTX 17 CIS + DOX IFOS + DOX 18 19 20 HDMTX 21 HDMTX 22 DOX CIS + DOX 23 1. Meyers PA et al. J Clin Oncol 2005; 23: 2004 -2011 2. Meyers PA et al. J Clin Oncol 2008; 26: 633 -638 Drug doses Cisplatin: 120 mg/m 2 four hours infusion Doxorubicin: 75 mg/m 2 72 hour continuous infusion High-dose methotrexate: 12 g/m 2 (max 20 g) four hour infusion followed by leucovorin 10 mg (fixed dose) 24 hours after start HDMTX continuing until serum high dose methotrexate < 1 x 10 -7 mol/L (100 nmol/L) Ifosfamide: 1. 8 g/m 2 for five days (total dose 9 g/m 2 (with mesna uroprotection) Mepact: 2 mg/m 2, with an additional 1 mg or 2 mg (maximum) at subsequent dosing according to patient biologic activity CIS: cisplatin; DOX: doxorubicin; HDMTX: high-dose methotrexate; IFOS: ifosfamide EUCAN/MEP/2013 -10019 September 2013

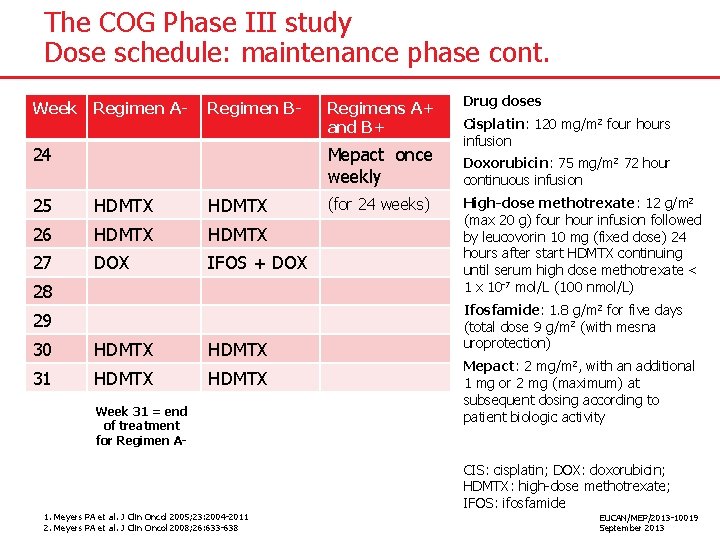
The COG Phase III study Dose schedule: maintenance phase cont. Week Regimen A- Regimen B- 24 Regimens A+ and B+ Mepact once weekly 25 HDMTX 26 HDMTX 27 DOX IFOS + DOX 28 29 30 HDMTX 31 HDMTX Week 31 = end of treatment for Regimen A- 1. Meyers PA et al. J Clin Oncol 2005; 23: 2004 -2011 2. Meyers PA et al. J Clin Oncol 2008; 26: 633 -638 (for 24 weeks) Drug doses Cisplatin: 120 mg/m 2 four hours infusion Doxorubicin: 75 mg/m 2 72 hour continuous infusion High-dose methotrexate: 12 g/m 2 (max 20 g) four hour infusion followed by leucovorin 10 mg (fixed dose) 24 hours after start HDMTX continuing until serum high dose methotrexate < 1 x 10 -7 mol/L (100 nmol/L) Ifosfamide: 1. 8 g/m 2 for five days (total dose 9 g/m 2 (with mesna uroprotection) Mepact: 2 mg/m 2, with an additional 1 mg or 2 mg (maximum) at subsequent dosing according to patient biologic activity CIS: cisplatin; DOX: doxorubicin; HDMTX: high-dose methotrexate; IFOS: ifosfamide EUCAN/MEP/2013 -10019 September 2013

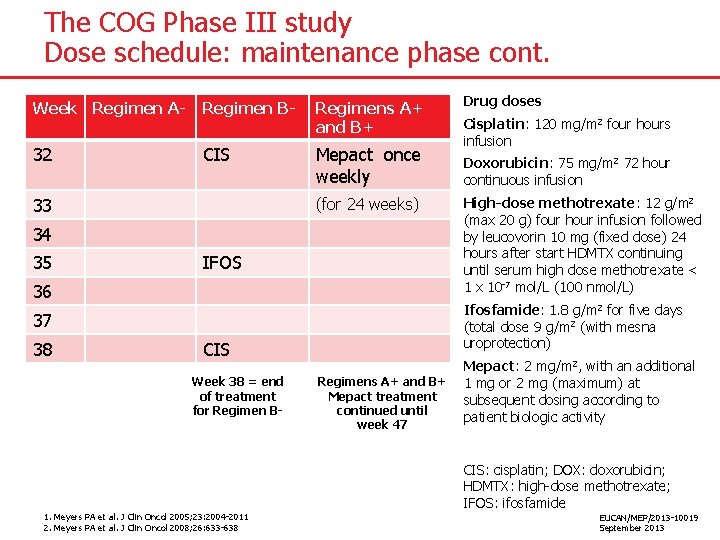
The COG Phase III study Dose schedule: maintenance phase cont. Week Regimen A- Regimen B- Regimens A+ and B+ 32 CIS Mepact once weekly 33 (for 24 weeks) 34 35 IFOS 36 CIS Week 38 = end of treatment for Regimen B- 1. Meyers PA et al. J Clin Oncol 2005; 23: 2004 -2011 2. Meyers PA et al. J Clin Oncol 2008; 26: 633 -638 Cisplatin: 120 mg/m 2 four hours infusion Doxorubicin: 75 mg/m 2 72 hour continuous infusion High-dose methotrexate: 12 g/m 2 (max 20 g) four hour infusion followed by leucovorin 10 mg (fixed dose) 24 hours after start HDMTX continuing until serum high dose methotrexate < 1 x 10 -7 mol/L (100 nmol/L) Ifosfamide: 1. 8 g/m 2 for five days (total dose 9 g/m 2 (with mesna uroprotection) 37 38 Drug doses Regimens A+ and B+ Mepact treatment continued until week 47 Mepact: 2 mg/m 2, with an additional 1 mg or 2 mg (maximum) at subsequent dosing according to patient biologic activity CIS: cisplatin; DOX: doxorubicin; HDMTX: high-dose methotrexate; IFOS: ifosfamide EUCAN/MEP/2013 -10019 September 2013

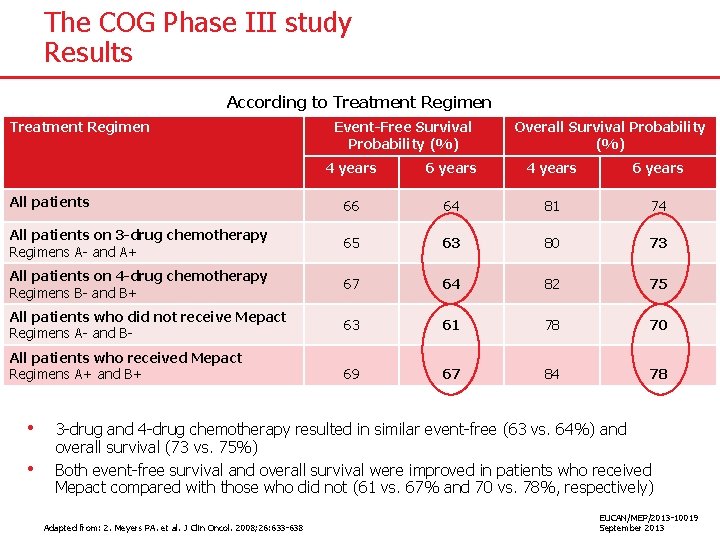
The COG Phase III study Results According to Treatment Regimen Event-Free Survival Probability (%) Overall Survival Probability (%) 4 years 6 years All patients 66 64 81 74 All patients on 3 -drug chemotherapy Regimens A- and A+ 65 63 80 73 All patients on 4 -drug chemotherapy Regimens B- and B+ 67 64 82 75 All patients who did not receive Mepact Regimens A- and B- 63 61 78 70 69 67 84 78 All patients who received Mepact Regimens A+ and B+ • • 3 -drug and 4 -drug chemotherapy resulted in similar event-free (63 vs. 64%) and overall survival (73 vs. 75%) Both event-free survival and overall survival were improved in patients who received Mepact compared with those who did not (61 vs. 67% and 70 vs. 78%, respectively) Adapted from: 2. Meyers PA. et al. J Clin Oncol. 2008; 26: 633 -638 EUCAN/MEP/2013 -10019 September 2013

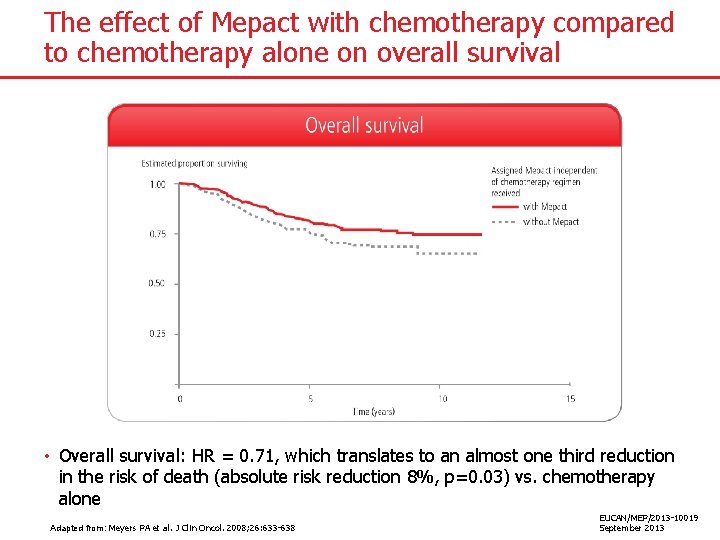
The effect of Mepact with chemotherapy compared to chemotherapy alone on overall survival • Overall survival: HR = 0. 71, which translates to an almost one third reduction in the risk of death (absolute risk reduction 8%, p=0. 03) vs. chemotherapy alone Adapted from: Meyers PA et al. J Clin Oncol. 2008; 26: 633 -638 EUCAN/MEP/2013 -10019 September 2013

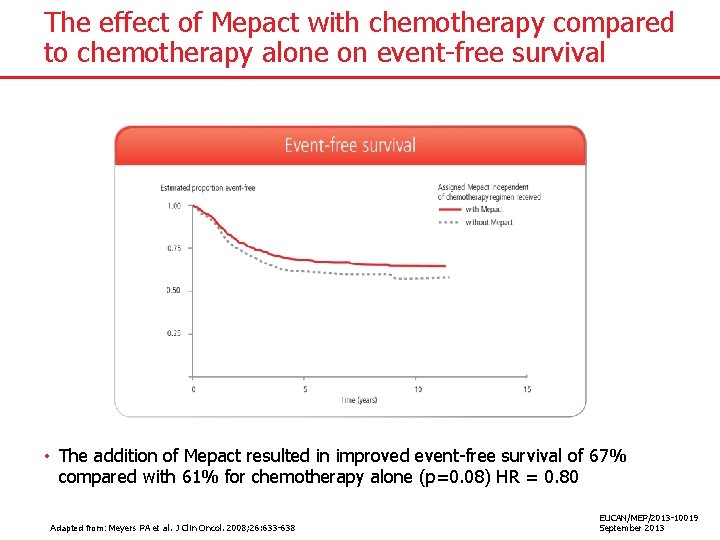
The effect of Mepact with chemotherapy compared to chemotherapy alone on event-free survival • The addition of Mepact resulted in improved event-free survival of 67% compared with 61% for chemotherapy alone (p=0. 08) HR = 0. 80 Adapted from: Meyers PA et al. J Clin Oncol. 2008; 26: 633 -638 EUCAN/MEP/2013 -10019 September 2013

Results of the Mepact with our without multi-agent chemotherapy on overall survival ● The addition of Mepact to chemotherapy: – Improved 6 -year overall survival from 70% to 78% (p=0. 03) – Improved 6 -year event-free survival from 61% to 67% (p=0. 08) – Reduced the risk of death by almost one third (relative risk=0. 71, absolute risk reduction 8%) Meyers PA. et al. J Clin Oncol. 2008; 26: 633 -638 EUCAN/MEP/2013 -10019 September 2013

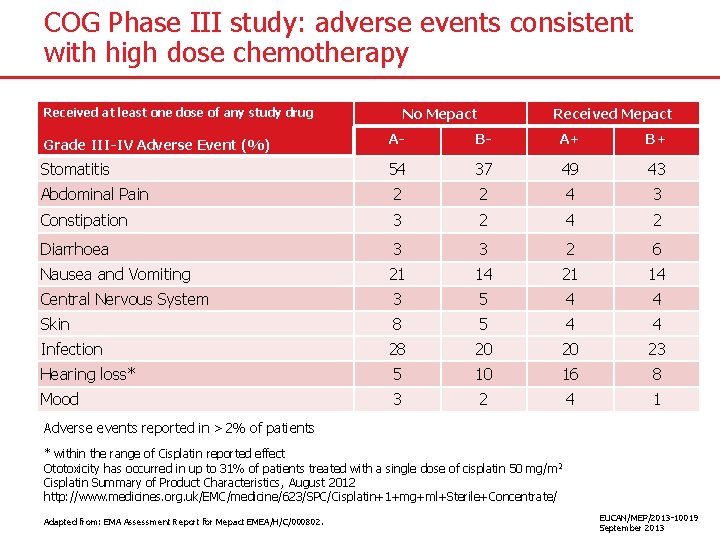
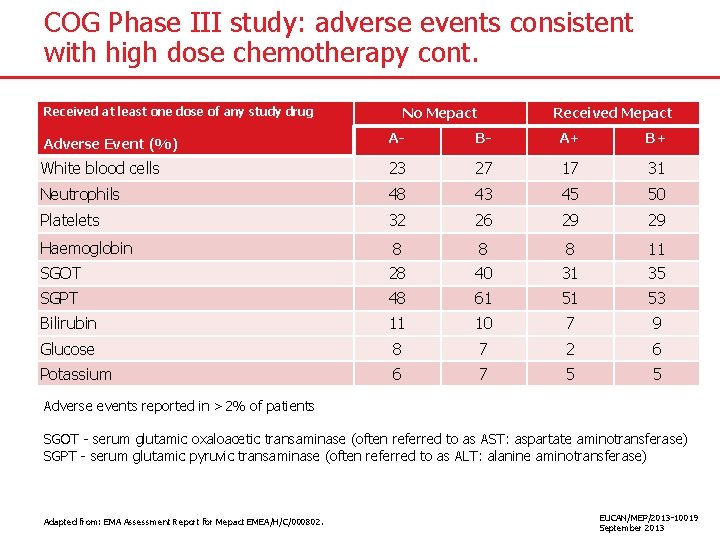
COG Phase III study: adverse events consistent with high dose chemotherapy No Mepact Received at least one dose of any study drug Received Mepact Grade III-IV Adverse Event (%) A- B- A+ B+ Stomatitis 54 37 49 43 Abdominal Pain 2 2 4 3 Constipation 3 2 4 2 Diarrhoea 3 3 2 6 Nausea and Vomiting 21 14 Central Nervous System 3 5 4 4 Skin 8 5 4 4 Infection 28 20 20 23 Hearing loss* 5 10 16 8 Mood 3 2 4 1 Adverse events reported in >2% of patients * within the range of Cisplatin reported effect Ototoxicity has occurred in up to 31% of patients treated with a single dose of cisplatin 50 mg/m 2 Cisplatin Summary of Product Characteristics, August 2012 http: //www. medicines. org. uk/EMC/medicine/623/SPC/Cisplatin+1+mg+ml+Sterile+Concentrate/ Adapted from: EMA Assessment Report for Mepact EMEA/H/C/000802. EUCAN/MEP/2013 -10019 September 2013

COG Phase III study: adverse events consistent with high dose chemotherapy cont. No Mepact Received at least one dose of any study drug Received Mepact Adverse Event (%) A- B- A+ B+ White blood cells 23 27 17 31 Neutrophils 48 43 45 50 Platelets 32 26 29 29 Haemoglobin 8 8 8 11 SGOT 28 40 31 35 SGPT 48 61 51 53 Bilirubin 11 10 7 9 Glucose 8 7 2 6 Potassium 6 7 5 5 Adverse events reported in >2% of patients SGOT - serum glutamic oxaloacetic transaminase (often referred to as AST: aspartate aminotransferase) SGPT - serum glutamic pyruvic transaminase (often referred to as ALT: alanine aminotransferase) Adapted from: EMA Assessment Report for Mepact EMEA/H/C/000802. EUCAN/MEP/2013 -10019 September 2013

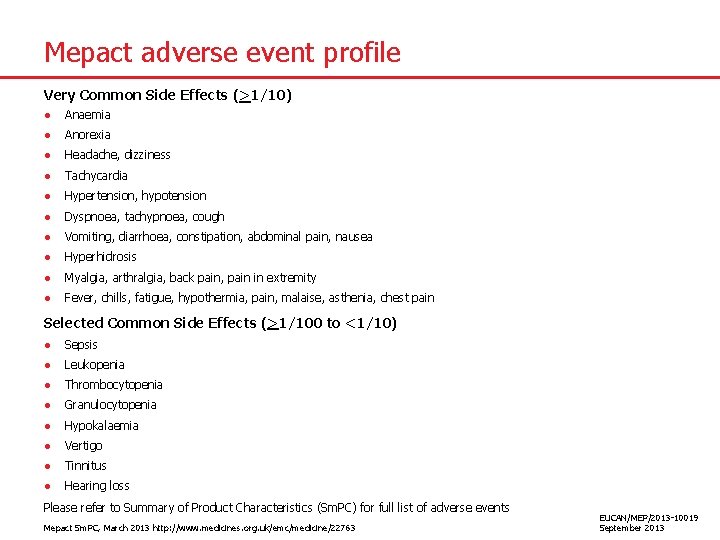
Mepact adverse event profile Very Common Side Effects (>1/10) ● Anaemia ● Anorexia ● Headache, dizziness ● Tachycardia ● Hypertension, hypotension ● Dyspnoea, tachypnoea, cough ● Vomiting, diarrhoea, constipation, abdominal pain, nausea ● Hyperhidrosis ● Myalgia, arthralgia, back pain, pain in extremity ● Fever, chills, fatigue, hypothermia, pain, malaise, asthenia, chest pain Selected Common Side Effects (>1/100 to <1/10) ● Sepsis ● Leukopenia ● Thrombocytopenia ● Granulocytopenia ● Hypokalaemia ● Vertigo ● Tinnitus ● Hearing loss Please refer to Summary of Product Characteristics (Sm. PC) for full list of adverse events Mepact Sm. PC, March 2013 http: //www. medicines. org. uk/emc/medicine/22763 EUCAN/MEP/2013 -10019 September 2013

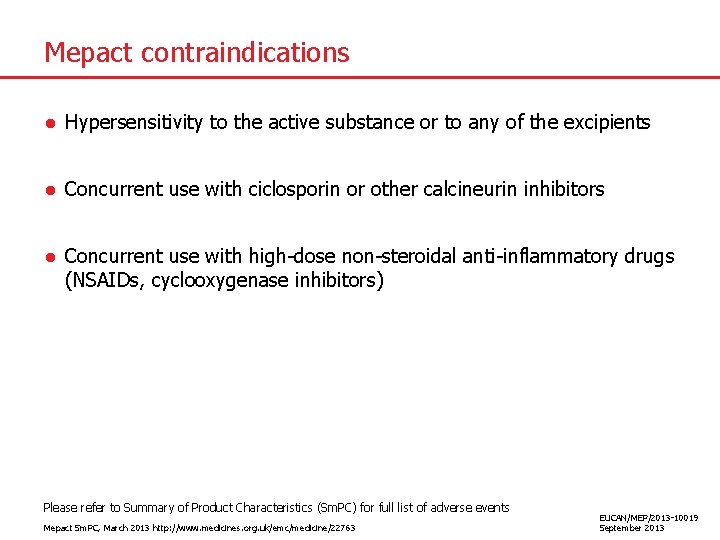
Mepact contraindications ● Hypersensitivity to the active substance or to any of the excipients ● Concurrent use with ciclosporin or other calcineurin inhibitors ● Concurrent use with high-dose non-steroidal anti-inflammatory drugs (NSAIDs, cyclooxygenase inhibitors) Please refer to Summary of Product Characteristics (Sm. PC) for full list of adverse events Mepact Sm. PC, March 2013 http: //www. medicines. org. uk/emc/medicine/22763 EUCAN/MEP/2013 -10019 September 2013

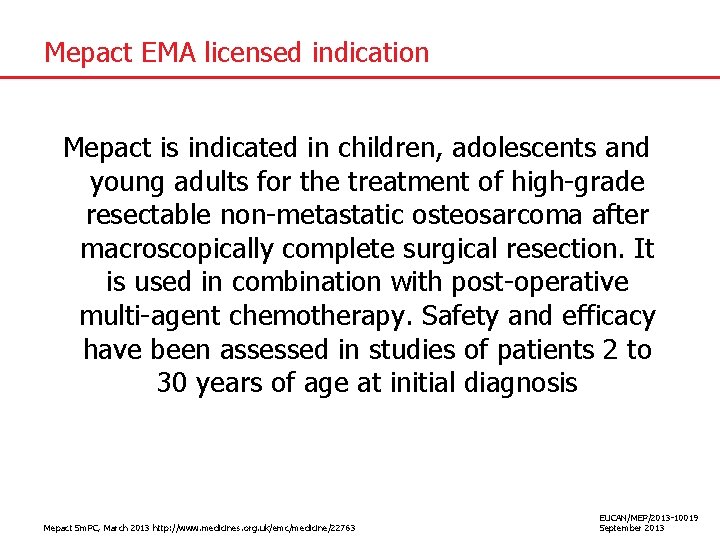
Mepact EMA licensed indication Mepact is indicated in children, adolescents and young adults for the treatment of high-grade resectable non-metastatic osteosarcoma after macroscopically complete surgical resection. It is used in combination with post-operative multi-agent chemotherapy. Safety and efficacy have been assessed in studies of patients 2 to 30 years of age at initial diagnosis Mepact Sm. PC, March 2013 http: //www. medicines. org. uk/emc/medicine/22763 EUCAN/MEP/2013 -10019 September 2013

MEPACT® Prescribing Information Please refer to Summary of Product Characteristics (Sm. PC) before prescribing Insert Local Prescribing Information and replace TPI Gmb. H (below)with LOC company name © 2013 – Takeda Pharmaceuticals International Gmb. H EUCAN/MEP/2013 -10019 September 2013
 Unmet financial need
Unmet financial need Icd 10 ca nasofaring
Icd 10 ca nasofaring Unmet needs in severe asthma
Unmet needs in severe asthma Self-fulfilling prophecy cycle
Self-fulfilling prophecy cycle Fulfilling obligations in a dependable and trustworthy way
Fulfilling obligations in a dependable and trustworthy way Self fulfilling prophecy example
Self fulfilling prophecy example Self fulfilling prophecy harry potter
Self fulfilling prophecy harry potter Basic human aspirations is
Basic human aspirations is Fulfilling lives in islington and camden
Fulfilling lives in islington and camden Self fulfilling prophecy sociology
Self fulfilling prophecy sociology Social inhibition example
Social inhibition example Statuses and their related roles determine
Statuses and their related roles determine Cognitive psychology concepts
Cognitive psychology concepts Self fulfilling prophecy sociology
Self fulfilling prophecy sociology How a self-fulfilling stereotype can drag down performance
How a self-fulfilling stereotype can drag down performance Self fulfilling prophecy psychology
Self fulfilling prophecy psychology You only need to be lucky once
You only need to be lucky once Research paper purpose
Research paper purpose A friend in need somerset maugham
A friend in need somerset maugham Why do plants need roots
Why do plants need roots Everything you need to know about the odyssey
Everything you need to know about the odyssey Why do we need to breathe
Why do we need to breathe I need some fire
I need some fire Verb + object + infinitive or gerund
Verb + object + infinitive or gerund Define comparative education
Define comparative education Prostitution types
Prostitution types How to calculate need matrix in banker's algorithm
How to calculate need matrix in banker's algorithm Dimension of channel design
Dimension of channel design Need of water purification
Need of water purification Unsatisfied need tension x
Unsatisfied need tension x Need for scientific selection
Need for scientific selection What is the highest level of human need according to maslow
What is the highest level of human need according to maslow Hebrews 10:36 sermon
Hebrews 10:36 sermon Why do all organisms need food
Why do all organisms need food Broad generalization
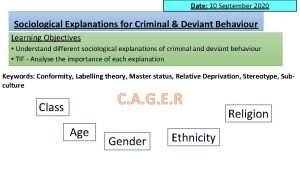
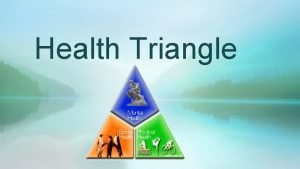
Broad generalization What are the 3 parts of the health triangle?
What are the 3 parts of the health triangle? Find market n
Find market n The space between traffic clusters is called
The space between traffic clusters is called How do animals get the nitrogen they need
How do animals get the nitrogen they need Why do we need food
Why do we need food Where to sign passport
Where to sign passport Child in need threshold
Child in need threshold George buckley harvard
George buckley harvard