Measurement in Science Learning Target 20 I can






















- Slides: 32

Measurement in Science

Learning Target #20 • I can differentiate between these scientific units of measurement: mass, volume, and weight.

Learning Target #21 • I can utilize the relationship between a substance’s mass and volume to determine its density.

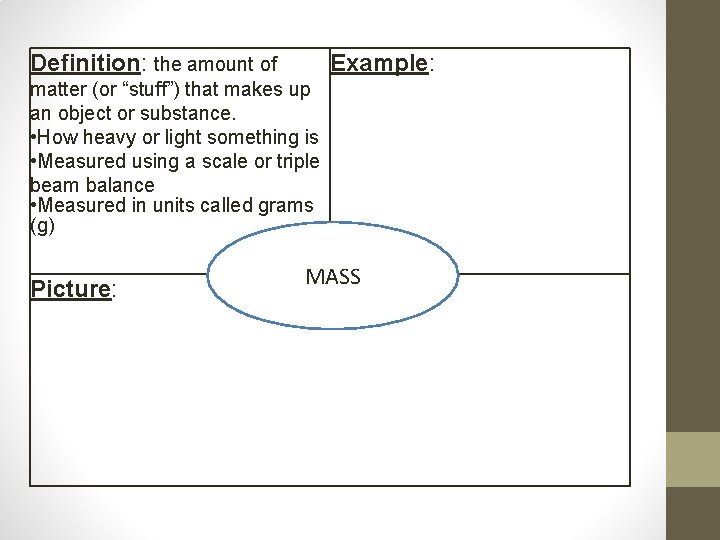
Mass • Mass- the amount of matter (or stuff) that makes up an object • Measured using a scale or triple beam balance • Measured in units called grams (g) Mass Versus Weight ***Mass is not the same as weight. *** • Weight is the force of gravity pulling on an object. • Weight can change based on your location but mass never changes • (Ex. You weigh 6 times more on Earth than you would on the moon… 100 lbs on Earth = 16 lbs on the moon!)

Definition: the amount of Example: matter (or “stuff”) that makes up an object or substance. • How heavy or light something is • Measured using a scale or triple beam balance • Measured in units called grams (g) Picture: MASS

Definition: the amount of Example: force of gravity pulling Your weight on the moon is 6 times less than what it is on an object on Earth • Measured in units called Newtons (N) • Weight changes based upon your location Picture: WEIGHT

Triple Beam Balance Scale: Getting Accurate Measurements (Calibration) • Calibrate the scale by sliding all three metal sliders to their leftmost positions. • Twist the zeroing screw (below the pan) until the balance pointer lines up with the fixed zero mark. • Twist forward– Pointer goes down • Twist backward- Pointer goes up

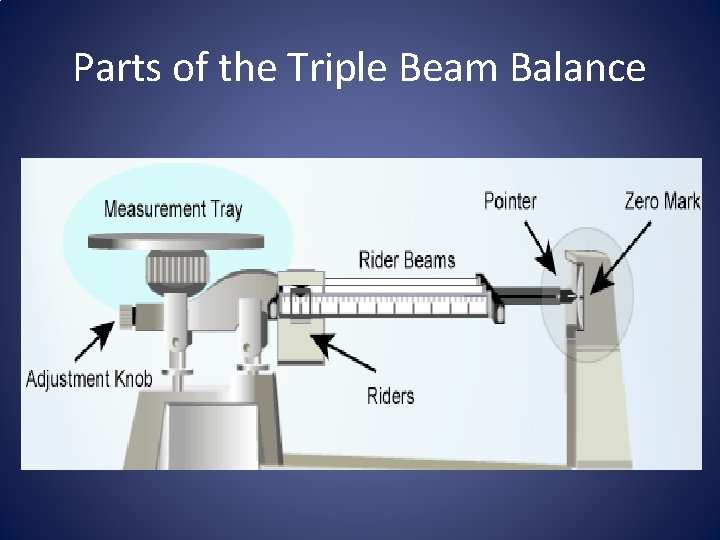
Parts of the Triple Beam Balance

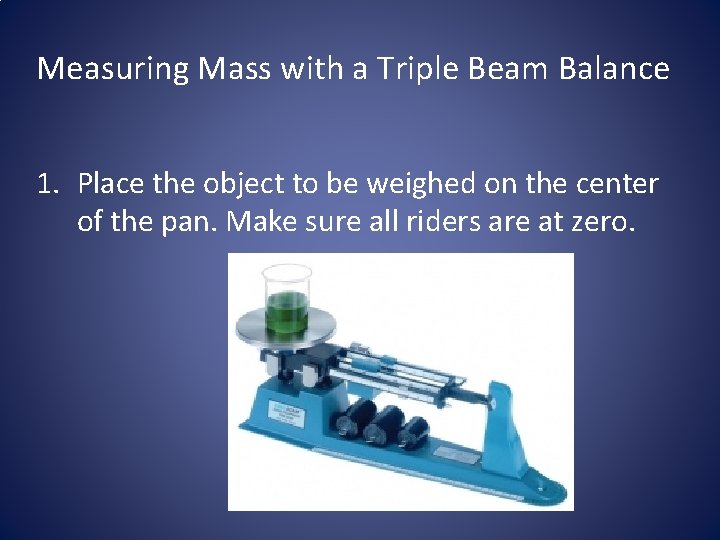
Measuring Mass with a Triple Beam Balance 1. Place the object to be weighed on the center of the pan. Make sure all riders are at zero.

100 -Gram Beam (Middle Beam) 2. Slide the 100 -gram rider right one notch at a time. When the pointer line drops below the fixed zero mark, move the rider back (to the left) one notch.

10 -Gram Beam (Top Beam) 3. Slide the 10 -gram rider right one notch at a time. Repeat the same steps as for the 100 Gram beam.

1 -Gram Beam (Bottom Beam) 4. Slide the 1 -gram rider slowly across the third beam (you might want to use your pencil). Stop sliding when the pointer lines up with the fixed zero mark. 5. Each little notch between a whole number represents 1/10 th. – Ex. If the pointer lines up after the 6, plus 4 more little notches, the total is 6. 4 g for that beam.

6. Add the values of all three beams to determine the mass of your object.

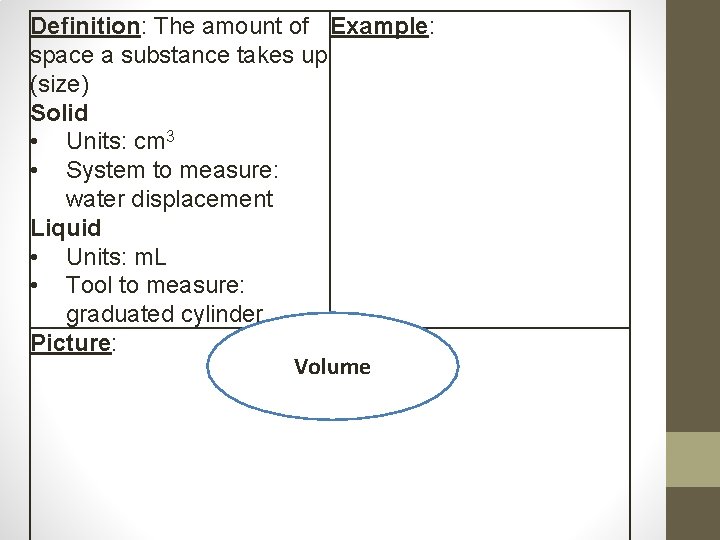
Volume • Volume- the amount of space an object or substance takes up (size) Tools to Measure Volume • Volume of an odd-shaped object is measured using a water displacement • Volume of a liquid is measured using a graduated cylinder or beaker Units for Volume • Liquid = milliliters (m. L) • Solid = cubic centimeters (cm 3)

Definition: The amount of Example: space a substance takes up (size) Solid • Units: cm 3 • System to measure: water displacement Liquid • Units: m. L • Tool to measure: graduated cylinder Picture: Volume

Example • Which has more volume? This Styrofoam block or this brick? Why?

Situation • Bo gets into a bathtub filled to the top with water. What will happen? Why?

Volume of a Liquid: Using a Graduated Cylinder Reading a graduated cylinder: 1. Determine the value of each mark on the graduated cylinder. 2. Read the lowest part of the meniscus of the liquid • Meniscus- the lowest part of the dip of the liquid as it sits in the graduated cylinder

Volume of an Odd Shaped Object: Using Water Displacement 1. Fill the graduated cylinder with water. Record the amount of water after reading the meniscus. 2. Carefully place the object into the water. Don’t splash (this could mess up your results)! Make sure that it is fully under the water. 3. Measure the new water level at the meniscus. 4. Subtract (new water level – old water level) 5. Your answer is the volume of the object.

Water Displacement Example 1 • Starting water volume? • Ending water volume? • Volume of the dinosaur toy?

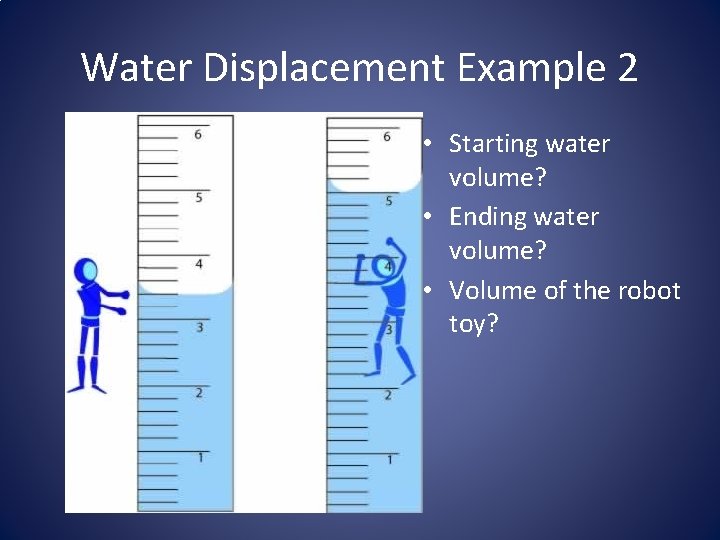
Water Displacement Example 2 • Starting water volume? • Ending water volume? • Volume of the robot toy?

Density • Density- the amount of mass something has compared to its volume • An easy way to think of it: The amount of “stuff” or matter packed into a certain area. Units for Measuring Density • g/cm 3 (solid) • g/m. L (liquid)

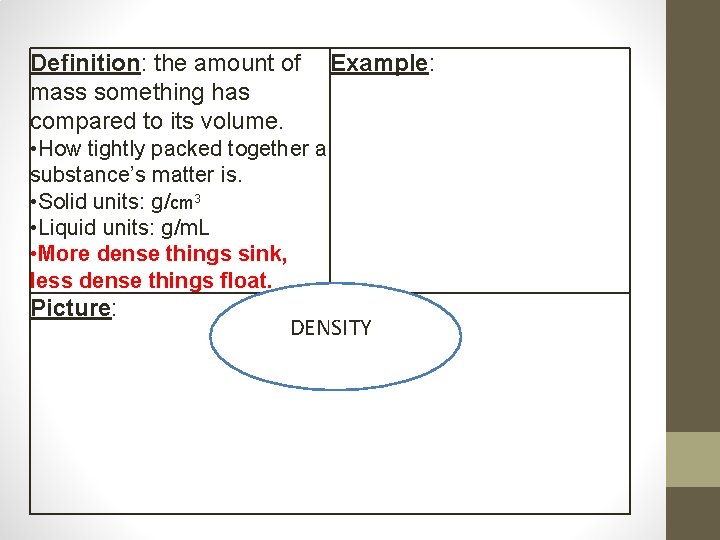
Definition: the amount of mass something has compared to its volume. Example: • How tightly packed together a substance’s matter is. • Solid units: g/cm 3 • Liquid units: g/m. L • More dense things sink, less dense things float. Picture: DENSITY

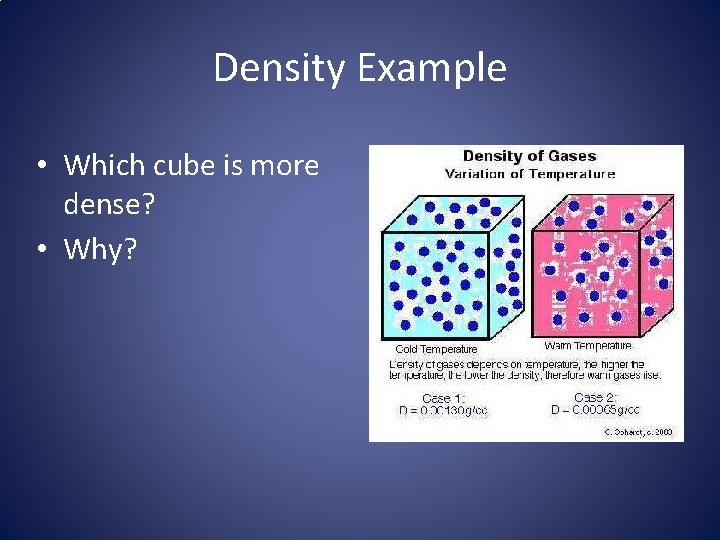
Density Example • Which cube is more dense? • Why?

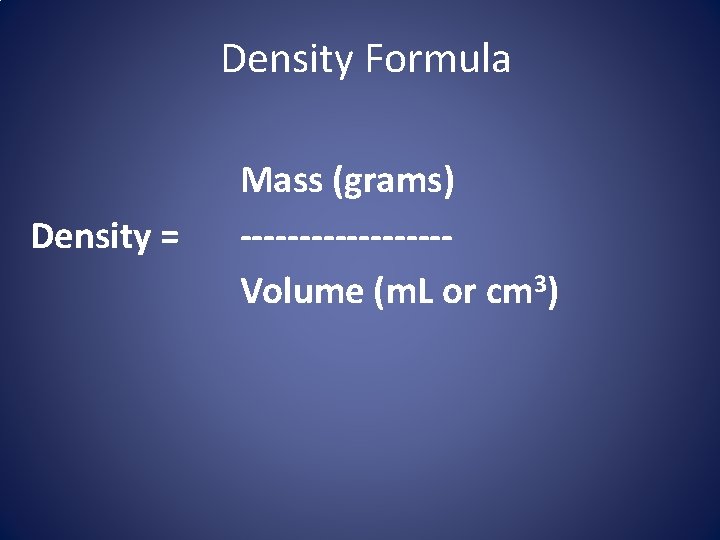
Density Formula Density = Mass (grams) ---------Volume (m. L or cm 3)

Practice Density Problems 1. A piece of metal has a volume of 76 cm 3 and a mass of 554. 8 g. Find the Density.

Density Lab Conclusions • Even if 2 of the SAME substances have different masses and volumes, they will have the same density. • Each substance has its own unique density, which can help people tell it apart from other substances. EX. ANY piece of pure Silver will have a density of 10. 5 g/cm 3

Archimedes Conundrum • Using what you know about mass, volume, and the density of different substances, describe how Archimedes could determine if the King’s crown was made entirely of gold.

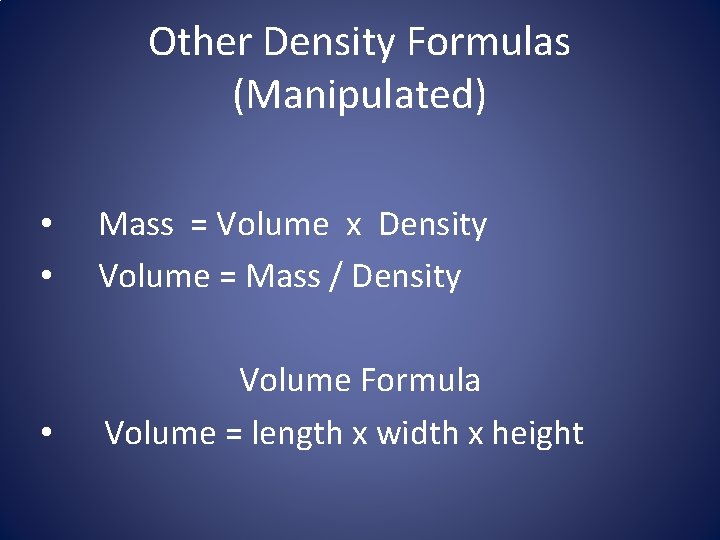
Other Density Formulas (Manipulated) • • Mass = Volume x Density Volume = Mass / Density • Volume Formula Volume = length x width x height

Mass = ? , Volume = 17. 3 m. L, Density = 34 g/m. L

Mass = 12 g, Volume = ? , Density = 64. 7 g/m. L

Mass = 24 g, Volume = 15. 2 m. L, Density = ?