MD Studies of LDL and HDL phases of





- Slides: 13

MD Studies of LDL and HDL phases of Supercooled Water Xiaohai Li (Ch. BE) Vamsi Akkineni (PHYS) Jianwei Wang (GEOL)

Project Goals • Use Molecular Dynamics to simulate the LDL and HDL phases of supercooled water • Obtain the radial distribution functions of two liquid phases of water • Compare Results with literature results

Motivation • The liquid-liquid phase transition hypothesis is difficult to prove experimentally because of homogeneous necleation • Computer simulation becomes extremely useful in this case because nucleation does not occur on the timescale of computer simulation

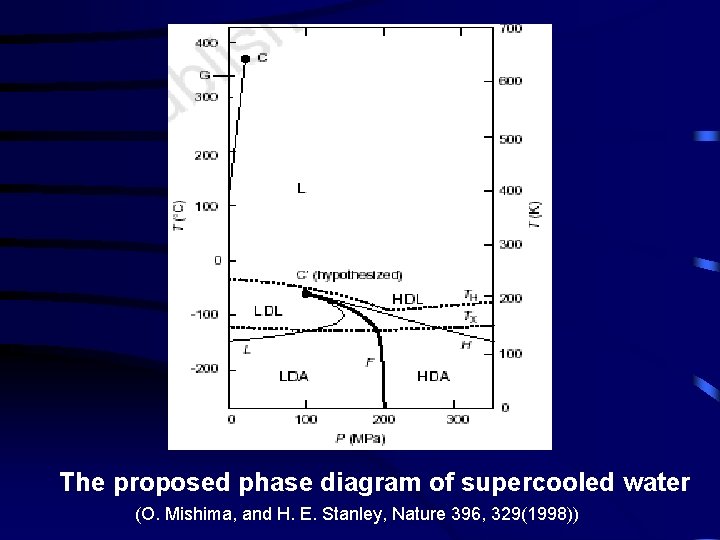
The proposed phase diagram of supercooled water (O. Mishima, and H. E. Stanley, Nature 396, 329(1998))

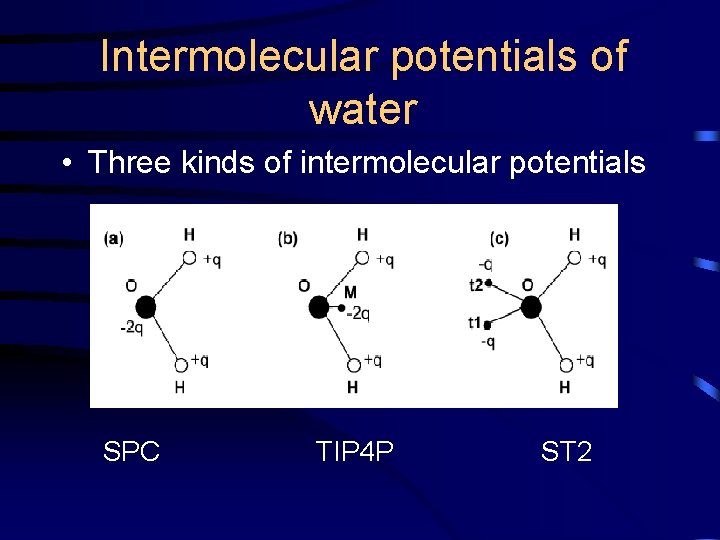
Intermolecular Potentials of water • Interactions between water molecules are far more complicated than those between particles of simple liquids – The ability of water molecules to form hydrogen bonds – The existence of substantial non-additive threeand high-body terms • The simulation are highly sensitive to the intermolecular potential functions used

Intermolecular potentials of water • Three kinds of intermolecular potentials SPC TIP 4 P ST 2

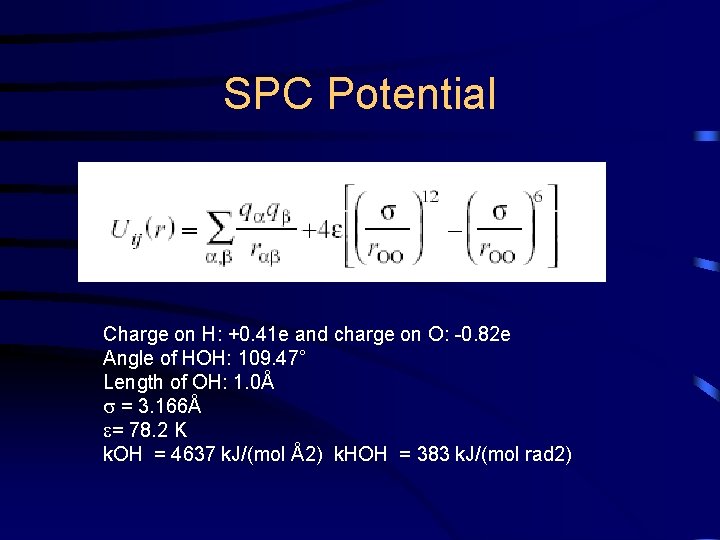
SPC Potential Charge on H: +0. 41 e and charge on O: -0. 82 e Angle of HOH: 109. 47° Length of OH: 1. 0Å = 3. 166Å = 78. 2 K k. OH = 4637 k. J/(mol Å2) k. HOH = 383 k. J/(mol rad 2)

MD Simulation • • Ensemble: NVT Number of water molecules: 125 Use periodic boundry conditions Time step: 0. 001 ps Equilibration: 500 ps Collect data: 1000 ps Ewald summation used for long range Coulombic forces. • Cutoff used for Lennard-Jones interactions (7Å)

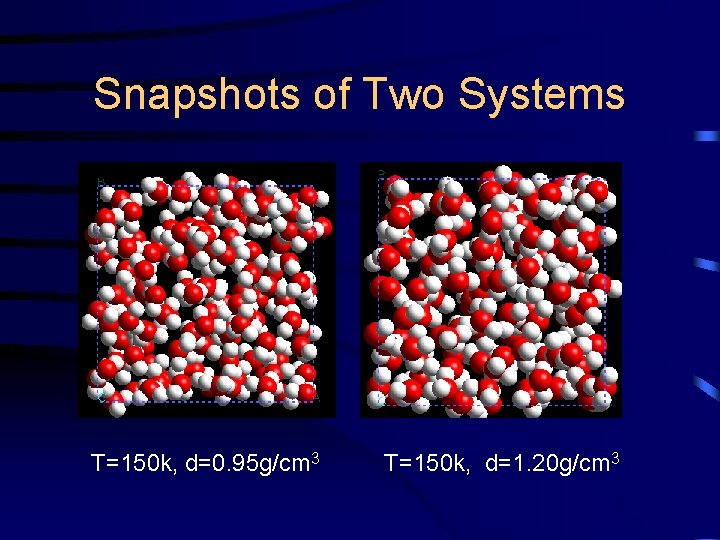
Snapshots of Two Systems T=150 k, d=0. 95 g/cm 3 T=150 k, d=1. 20 g/cm 3


(Huang et al. )

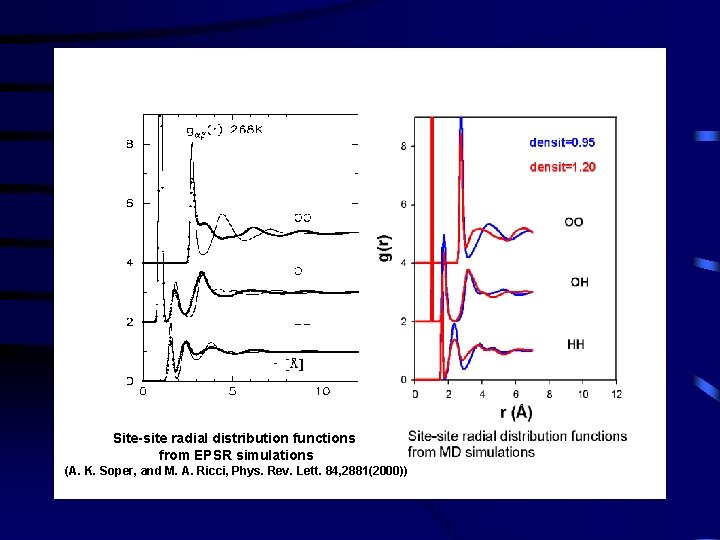
Site-site radial distribution functions from EPSR simulations (A. K. Soper, and M. A. Ricci, Phys. Rev. Lett. 84, 2881(2000))

Conclusion • SPC model is suitable for simulating the supercooled water • Our simulation supports the conjecture of LDL and HDL phases at low temperature • More state points can be simulated to find the second critical point