MATERIALS Types of materials Metals and non metals










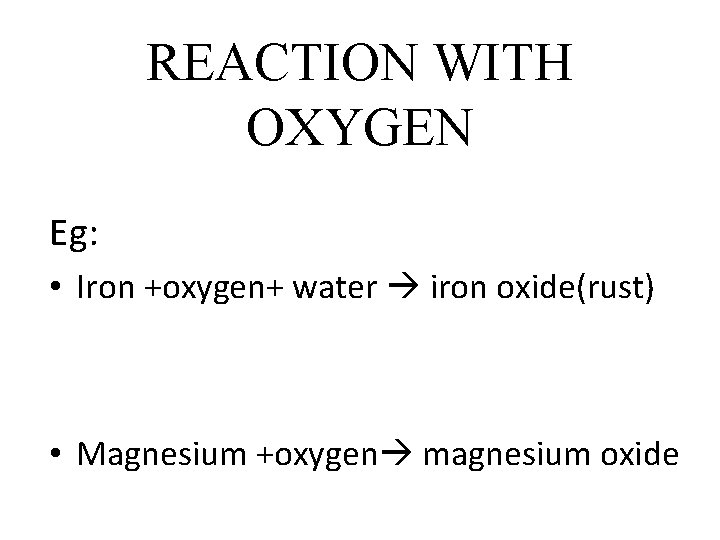


- Slides: 13

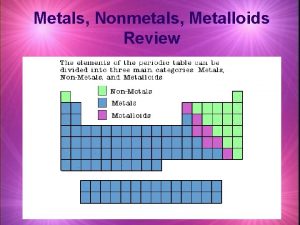
MATERIALS • Types of materials: Metals and non metals. • Physical properties of metals: • Metals are lustrous, sonorous, malleable, ductile, good conductors of heat and electricty.

LUSTROUS • Bright appearance. • Ductility: Metals can be drawn in to wires. • Sonorous: Producing sound.

MALLEABILITY • Metals are lustrous, sonorous, malleable, • ductile, good conductors of heat and • Ductility: Metals can be drawn in to wires. • Sonorous: Producing sound.

GOOD CONDUCTORS • Good conductors: Conduct heat and electricity. • Hard • Examples; Iron, copper, aluminum, magnesium etc

PHYSICAL PROPERTIES Non metals Non-sonorous Brittle Poor conductors of heat and electricity. • Non malleable. • •

NON METALS • They breakdown easily. • They are not sonorous. • They are poor conductors of heat and electricity.

EXAMPLES • Non- metals • sulphur, carbon, oxygen, phosphorous etc. • Exceptions: • Metals like sodium, potassium can • Cut with knife. • Mercury only metal in liquid state.

CHEMICAL PROPERTIES • • Rusting : Iron reacts with oxygen to form rust. Reaction of metals with oxygen. Metals react with oxygen to form its oxide.

REACTION OF NON– METALS WITH OXYGEN • Sulphur +oxygen sulphur di-oxide • When sulphur di-oxide is added with water, sulphurous acid is formed. • It turns blue litmus to red which shows that it is acidic in nature.

METAL OXIDE – BASIC IN NATURE • When magnesium oxide is added with water and tested with red litmus then red turns to blue, Which shows that it is basic in nature. • Mg. O + H 2 O Mg(OH)2
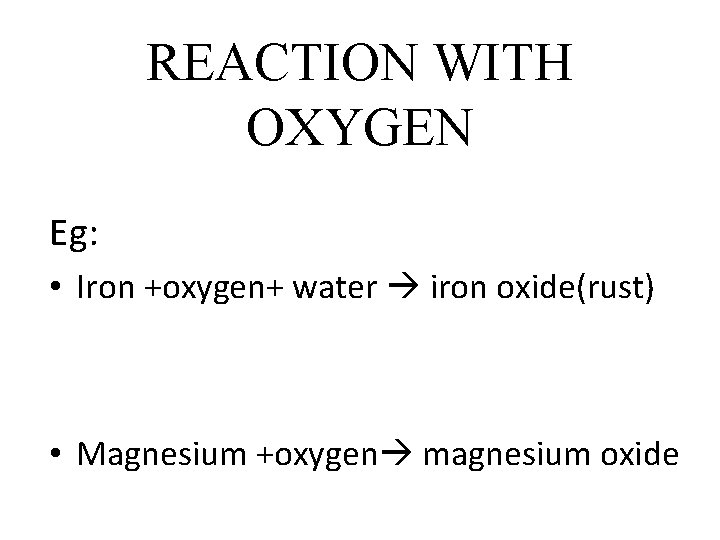
REACTION WITH OXYGEN Eg: • Iron +oxygen+ water iron oxide(rust) • Magnesium +oxygen magnesium oxide

SUMMARY • Metals are lustrous, malleable, ductile and good conductors • Non-metals are brittle, poor conductors, non lustrous etc • Metals react with oxygen to form its oxides • Metal oxide is basic in nature. • Non metal oxides are acidic in nature.

Thank you End of module II/III
 Metals metalloids and nonmetals periodic table
Metals metalloids and nonmetals periodic table Metals and non metals
Metals and non metals Properties of matter and materials grade 7
Properties of matter and materials grade 7 Matter and materials grade 7
Matter and materials grade 7 Examples of nonmetals
Examples of nonmetals Ferrous metals vs non ferrous metals
Ferrous metals vs non ferrous metals Un appel vient pour des soldats
Un appel vient pour des soldats Chapter 4 materials metals and nonmetals
Chapter 4 materials metals and nonmetals Melting point of diamond
Melting point of diamond Nonmetals uses
Nonmetals uses 5 non ferrous metals
5 non ferrous metals Metals vs nonmetals periodic table
Metals vs nonmetals periodic table Non metals
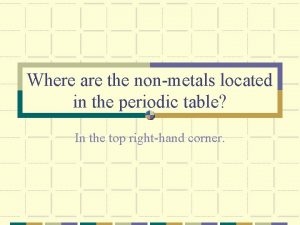
Non metals Where are nonmetals located on the periodic table?
Where are nonmetals located on the periodic table?