Manifestation of Sickle Cell Disease on Neuroimaging Neha









- Slides: 37

Manifestation of Sickle Cell Disease on Neuroimaging Neha Gowali, MD Gunja Parikh, MD Elana Smith, MD ASNR 2016 Annual Meeting e. Ed. E#: e. Ed. E-58 Control#: 1554

Disclosure of Commercial Interest Neither I nor my immediate family members have a financial relationship with a commercial organization that may have a direct or indirect interest on the content.

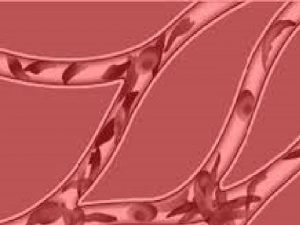
INTRODUCTION • Sickle cell disease (SCD) is a form of hemolytic anemia and is an inherited autosomal recessive disease. The disease entity can affect any organ system and therefore, the clinical presentation can vary. The name for the disease comes from the sickling of red blood cells (RBC) due to abnormal hemoglobin production. • Patients with SCD not only battle with chronic anemia but also suffer through two other manifestations of the disease: the vaso-occlusive process and the increased possibility of infections. There is significant morbidity and mortality associated with the disease and the neurological manifestations contribute to a major portion of it. • The aim of this presentation is to inform the audience of the different manifestations of the disease in the nervous system as evaluated by radiology.

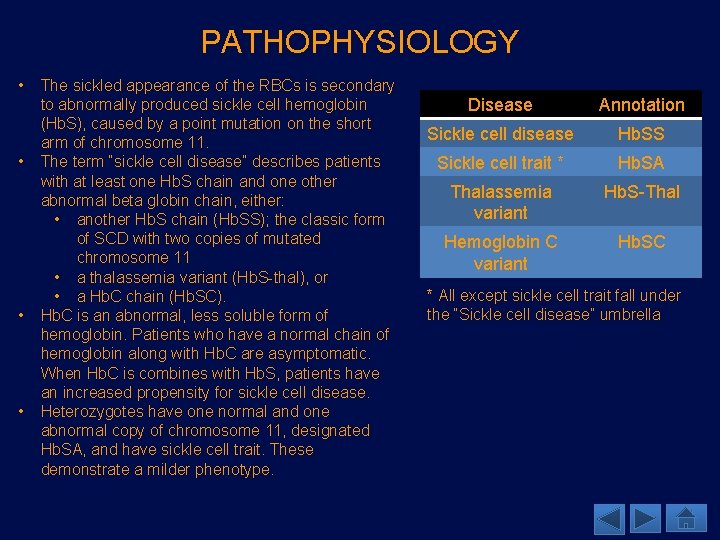
PATHOPHYSIOLOGY • • The sickled appearance of the RBCs is secondary to abnormally produced sickle cell hemoglobin (Hb. S), caused by a point mutation on the short arm of chromosome 11. The term “sickle cell disease” describes patients with at least one Hb. S chain and one other abnormal beta globin chain, either: • another Hb. S chain (Hb. SS); the classic form of SCD with two copies of mutated chromosome 11 • a thalassemia variant (Hb. S-thal), or • a Hb. C chain (Hb. SC). Hb. C is an abnormal, less soluble form of hemoglobin. Patients who have a normal chain of hemoglobin along with Hb. C are asymptomatic. When Hb. C is combines with Hb. S, patients have an increased propensity for sickle cell disease. Heterozygotes have one normal and one abnormal copy of chromosome 11, designated Hb. SA, and have sickle cell trait. These demonstrate a milder phenotype. Disease Annotation Sickle cell disease Hb. SS Sickle cell trait * Hb. SA Thalassemia variant Hb. S-Thal Hemoglobin C variant Hb. SC * All except sickle cell trait fall under the “Sickle cell disease” umbrella

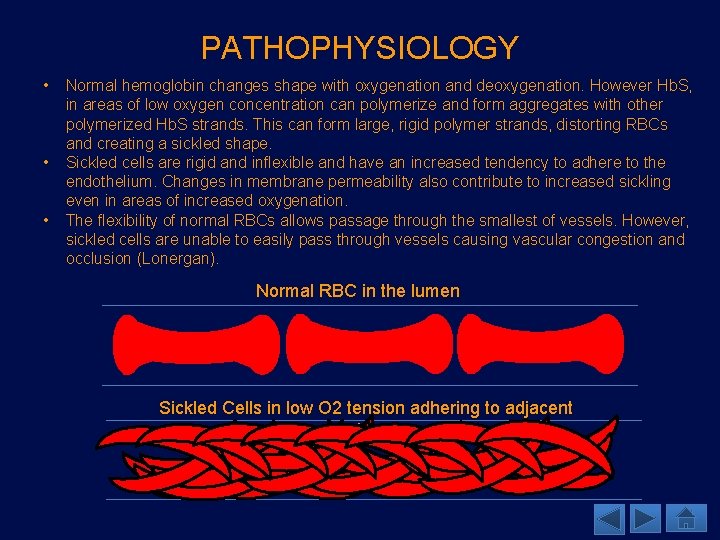
PATHOPHYSIOLOGY • • • Normal hemoglobin changes shape with oxygenation and deoxygenation. However Hb. S, in areas of low oxygen concentration can polymerize and form aggregates with other polymerized Hb. S strands. This can form large, rigid polymer strands, distorting RBCs and creating a sickled shape. Sickled cells are rigid and inflexible and have an increased tendency to adhere to the endothelium. Changes in membrane permeability also contribute to increased sickling even in areas of increased oxygenation. The flexibility of normal RBCs allows passage through the smallest of vessels. However, sickled cells are unable to easily pass through vessels causing vascular congestion and occlusion (Lonergan). Normal RBC in the lumen Sickled Cells in low O 2 tension adhering to adjacent endothelium

EPIDEMIOLOGY • SCD is predominantly found in patients of African descent. • It is also seen in Afro-Caribbean, Mediterranean, and Middle Eastern countries, as well as in the Indian subcontinent. • In the United States, about 8% of African Americans carry the Hb. S gene. • 1/600 African Americans are homozygous Hb. SS. • 60 – 70% sickle cell disease in the United States is secondary to Hb. SS (Lonergan). • Sickle cell trait (Hb. SA) is seen in ~1/12 African Americans. • Patients with sickle cell trait do not present with sickle cell disease, however, it is important to diagnose them for genetic counseling. • Patients with sickle cell trait have an increased risk of renal medullary carcinoma and reduced susceptibility to malaria (Lonergan).

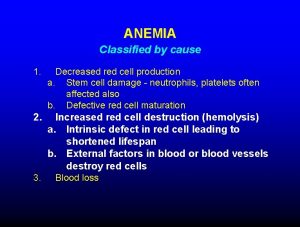
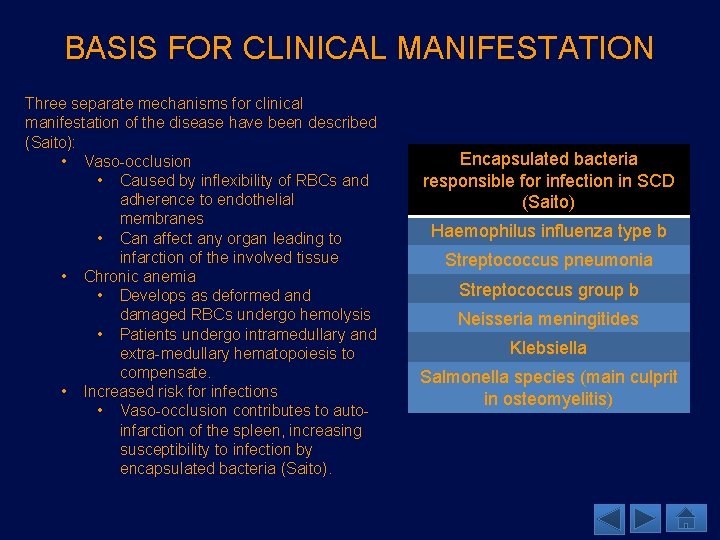
BASIS FOR CLINICAL MANIFESTATION Three separate mechanisms for clinical manifestation of the disease have been described (Saito): • Vaso-occlusion • Caused by inflexibility of RBCs and adherence to endothelial membranes • Can affect any organ leading to infarction of the involved tissue • Chronic anemia • Develops as deformed and damaged RBCs undergo hemolysis • Patients undergo intramedullary and extra-medullary hematopoiesis to compensate. • Increased risk for infections • Vaso-occlusion contributes to autoinfarction of the spleen, increasing susceptibility to infection by encapsulated bacteria (Saito). Encapsulated bacteria responsible for infection in SCD (Saito) Haemophilus influenza type b Streptococcus pneumonia Streptococcus group b Neisseria meningitides Klebsiella Salmonella species (main culprit in osteomyelitis)

CNS MANIFESTATIONS • Neurological manifestations of SCD range from silent ischemia, infarctions, and vasculopathies to intracranial hemorrhage. • 25% of all patients with SCD will suffer from its neurological manifestations in their lifetime. Eleven percent of complications have been said to occur before third decade (Lonergan). In children under ten years of age with SCD, the prevalence of infarction, silent ischemia and atrophy is approximately 22% (Thust). • Due to the varied presentations and neurological findings in patients with SCD, imaging plays an important role in diagnosis and follow up.

VASCULOPATHY • Vasculopathy is a major cause of neurological manifestations of SCD and involves both large and small vessels. • Distal internal carotid arteries, proximal anterior cerebral arteries (ACA) and middle cerebral arteries (MCA) are often involved. • Pathophysiology: • Intimal hyperplasia results in luminal narrowing causing sickled RBCs to adhere and cause sludging (Thust). • Stenosis of the main intracranial vessels leads to formation of numerous collateral vessels giving an appearance of Moyamoya. • Collaterals arise from the thalamoperforate and lenticulostriate arteries • Imaging findings: • On angiography, the vessels demonstrate a “puff of smoke” appearance along with occlusion or stenosis of the main intracranial vessels. • MR demonstrates numerous small flow voids in the basal ganglia representing collaterals (Lonergan). • Compensatory dilatation of pial vessels may also occur (Thust), and is best seen on FLAIR sequence as areas of increased signal in the region of leptomeningeal collaterals, suggestive of “ivy” draping the brain surface.

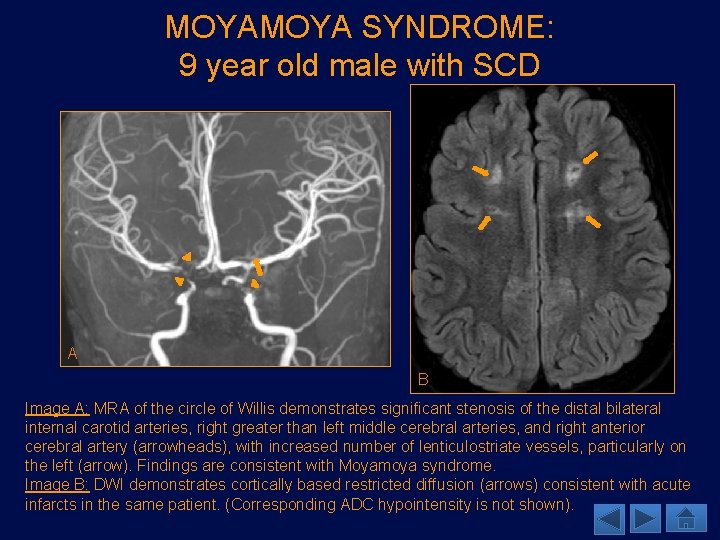
MOYA SYNDROME: 9 year old male with SCD A B Image A: MRA of the circle of Willis demonstrates significant stenosis of the distal bilateral internal carotid arteries, right greater than left middle cerebral arteries, and right anterior cerebral artery (arrowheads), with increased number of lenticulostriate vessels, particularly on the left (arrow). Findings are consistent with Moyamoya syndrome. Image B: DWI demonstrates cortically based restricted diffusion (arrows) consistent with acute infarcts in the same patient. (Corresponding ADC hypointensity is not shown).

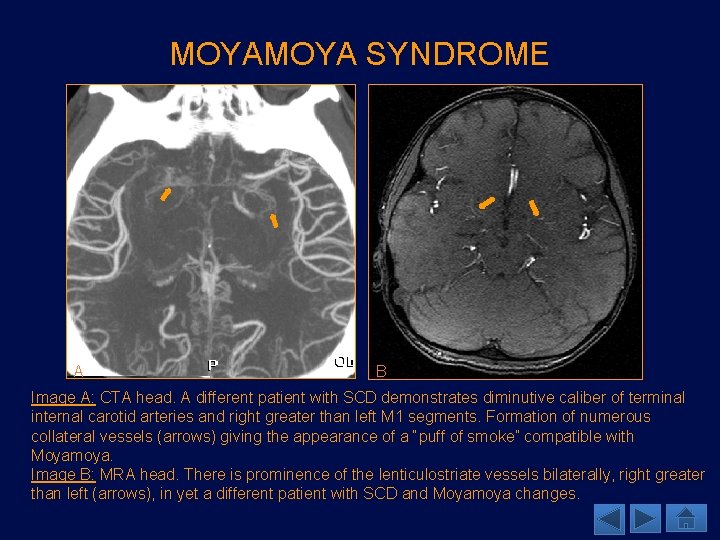
MOYA SYNDROME A B Image A: CTA head. A different patient with SCD demonstrates diminutive caliber of terminal internal carotid arteries and right greater than left M 1 segments. Formation of numerous collateral vessels (arrows) giving the appearance of a “puff of smoke” compatible with Moyamoya. Image B: MRA head. There is prominence of the lenticulostriate vessels bilaterally, right greater than left (arrows), in yet a different patient with SCD and Moyamoya changes.

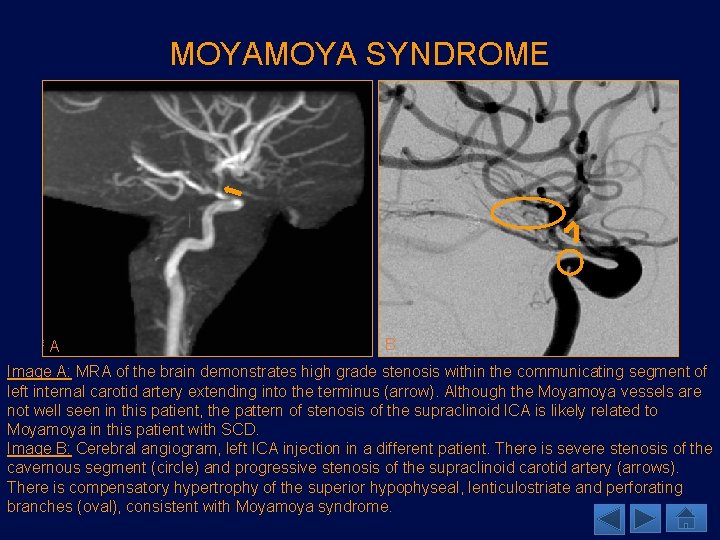
MOYA SYNDROME A B Image A: MRA of the brain demonstrates high grade stenosis within the communicating segment of left internal carotid artery extending into the terminus (arrow). Although the Moyamoya vessels are not well seen in this patient, the pattern of stenosis of the supraclinoid ICA is likely related to Moyamoya in this patient with SCD. Image B: Cerebral angiogram, left ICA injection in a different patient. There is severe stenosis of the cavernous segment (circle) and progressive stenosis of the supraclinoid carotid artery (arrows). There is compensatory hypertrophy of the superior hypophyseal, lenticulostriate and perforating branches (oval), consistent with Moyamoya syndrome.

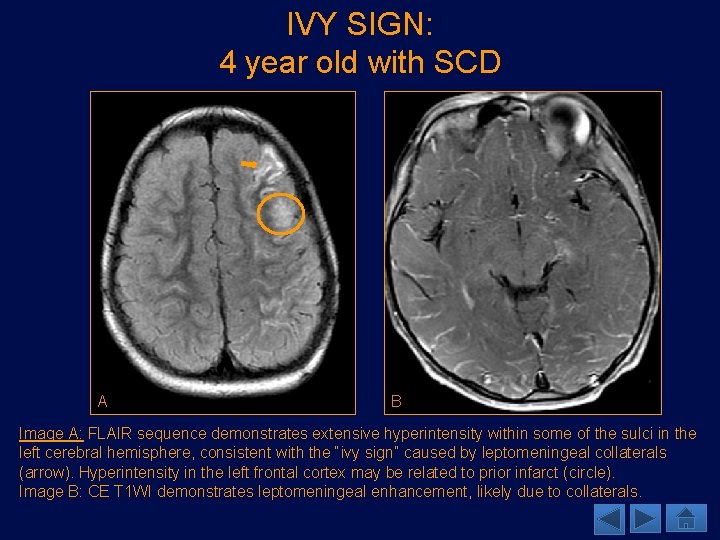
IVY SIGN: 4 year old with SCD A B Image A: FLAIR sequence demonstrates extensive hyperintensity within some of the sulci in the left cerebral hemisphere, consistent with the “ivy sign” caused by leptomeningeal collaterals (arrow). Hyperintensity in the left frontal cortex may be related to prior infarct (circle). Image B: CE T 1 WI demonstrates leptomeningeal enhancement, likely due to collaterals.

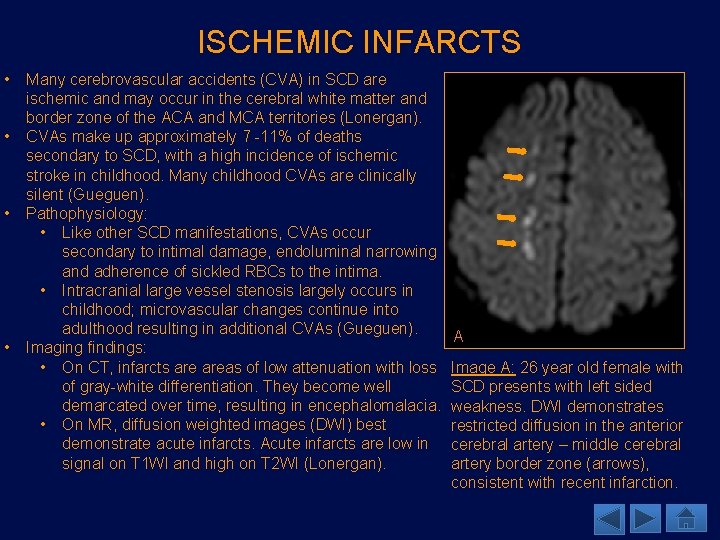
ISCHEMIC INFARCTS • • Many cerebrovascular accidents (CVA) in SCD are ischemic and may occur in the cerebral white matter and border zone of the ACA and MCA territories (Lonergan). CVAs make up approximately 7 -11% of deaths secondary to SCD, with a high incidence of ischemic stroke in childhood. Many childhood CVAs are clinically silent (Gueguen). Pathophysiology: • Like other SCD manifestations, CVAs occur secondary to intimal damage, endoluminal narrowing and adherence of sickled RBCs to the intima. • Intracranial large vessel stenosis largely occurs in childhood; microvascular changes continue into adulthood resulting in additional CVAs (Gueguen). Imaging findings: • On CT, infarcts areas of low attenuation with loss of gray-white differentiation. They become well demarcated over time, resulting in encephalomalacia. • On MR, diffusion weighted images (DWI) best demonstrate acute infarcts. Acute infarcts are low in signal on T 1 WI and high on T 2 WI (Lonergan). A Image A: 26 year old female with SCD presents with left sided weakness. DWI demonstrates restricted diffusion in the anterior cerebral artery – middle cerebral artery border zone (arrows), consistent with recent infarction.

ISCHEMIC INFARCTS • If there is cognitive impairment noted in children with SCD, it is important to search for underlying neurological causes. • Silent cerebral ischemia may occur in 27% of the patients with SCD before six years of age (Thust). Patients may present with normal findings on MR, however, may demonstrate decreased T 1 relaxation time in the gray and white matter on quantitative MR imaging (Lonergan). • Transcranial Doppler ultrasound has been used for evaluation of cerebral artery flow in patients with high risk of strokes. Lonergan et al report of studies demonstrating increased risk of strokes in patients with elevated velocities in the distal internal carotid arteries and proximal middle cerebral arteries. • Velocities less than 170 cm/sec are considered normal. • Velocities between 170 and 200 cm/sec demonstrate high risk. • Patients with two consecutive measurements of high velocity are recommended to have maintenance blood transfusion therapy, which has been known to reduce risks of strokes by 92% in children (Gueguen).

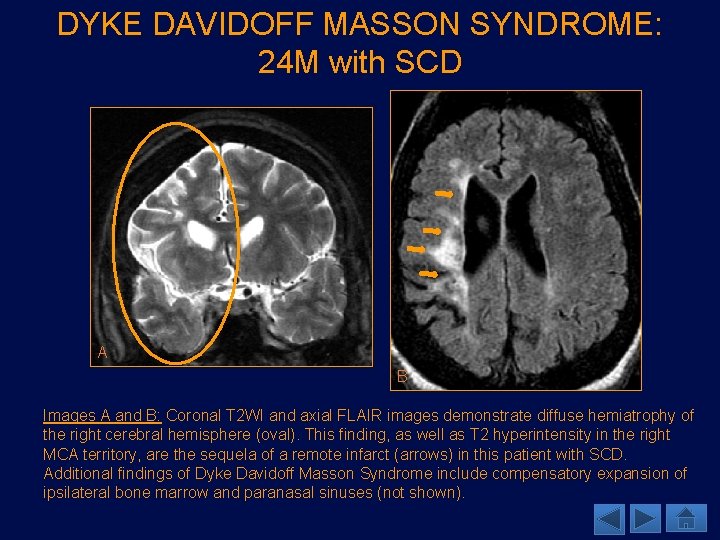
DYKE DAVIDOFF MASSON SYNDROME: 24 M with SCD A B Images A and B: Coronal T 2 WI and axial FLAIR images demonstrate diffuse hemiatrophy of the right cerebral hemisphere (oval). This finding, as well as T 2 hyperintensity in the right MCA territory, are the sequela of a remote infarct (arrows) in this patient with SCD. Additional findings of Dyke Davidoff Masson Syndrome include compensatory expansion of ipsilateral bone marrow and paranasal sinuses (not shown).

ANEURYSM FORMATION • Aneurysm formation is often seen in patients with SCD and is a common cause for subarachnoid hemorrhage (SAH) • Cerebral aneurysms in SCD differ from those in the general population. • Kossorotoff et al found that in general pediatric population, posterior circulation aneurysms are usually fusiform and anterior circulation aneurysms are saccular. The opposite is true in pediatric SCD population. • Aneurysm formation and CVA have similar pathophysiology. • Vessels undergo intimal hyperplasia, smooth muscle layer hyalinization and elastic lamina fragmentation (Kossorotoff), leading to stenosis due to increased scarring or dilatation from wall degeneration.

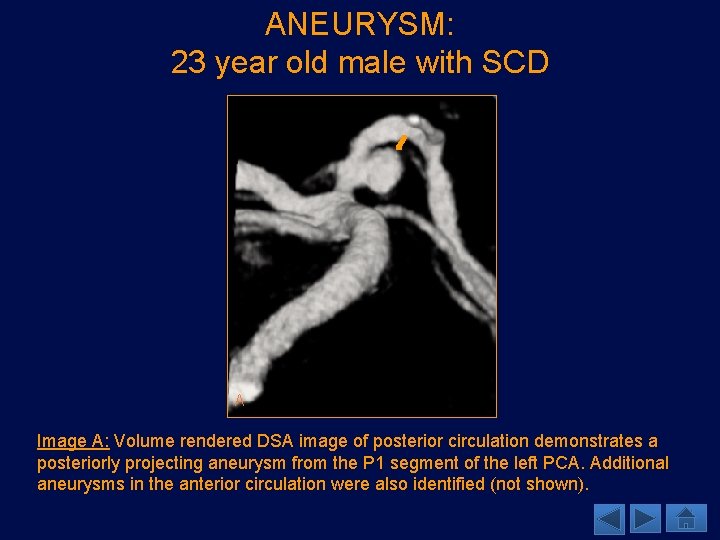
ANEURYSM: 23 year old male with SCD A Image A: Volume rendered DSA image of posterior circulation demonstrates a posteriorly projecting aneurysm from the P 1 segment of the left PCA. Additional aneurysms in the anterior circulation were also identified (not shown).

INTRACRANIAL HEMORRHAGE • Hemorrhagic stroke occurs at a lesser frequency than ischemia in children with SCD. In younger adults with SCD, however, hemorrhagic strokes predominate. • Hemorrhagic strokes are associated with higher risk of death than ischemic strokes (Gueguen). • According to Kossorotoff et al, risk factors common to both ischemic and hemorrhagic strokes include low hemoglobin concentration, high leukocyte count, acute chest syndrome, acute hypertension and hyper-transfusion, previous ischemic stroke and moya. • Ischemic and hemorrhagic strokes also share common pathophysiology. • Parenchymal and intraventricular hemorrhage occur secondary to rupture of collateral vessels that form due to intracranial vasculopathy (for example, the lenticulostriate vessels) (Thust).

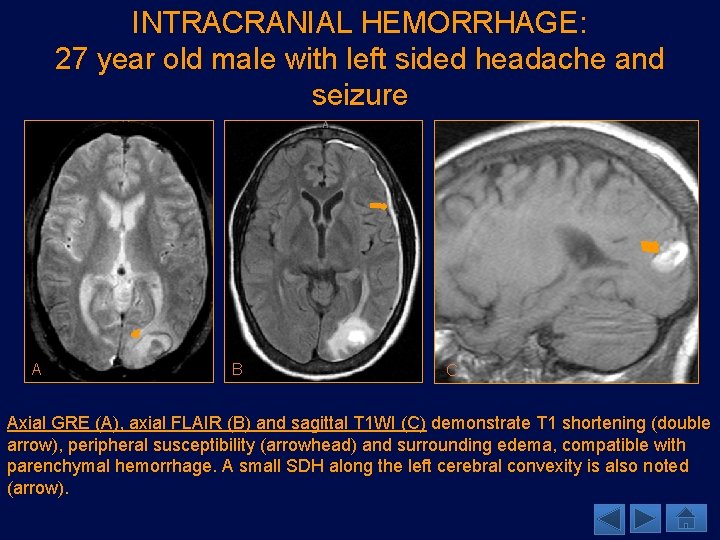
INTRACRANIAL HEMORRHAGE: 27 year old male with left sided headache and seizure A B C Axial GRE (A), axial FLAIR (B) and sagittal T 1 WI (C) demonstrate T 1 shortening (double arrow), peripheral susceptibility (arrowhead) and surrounding edema, compatible with parenchymal hemorrhage. A small SDH along the left cerebral convexity is also noted (arrow).

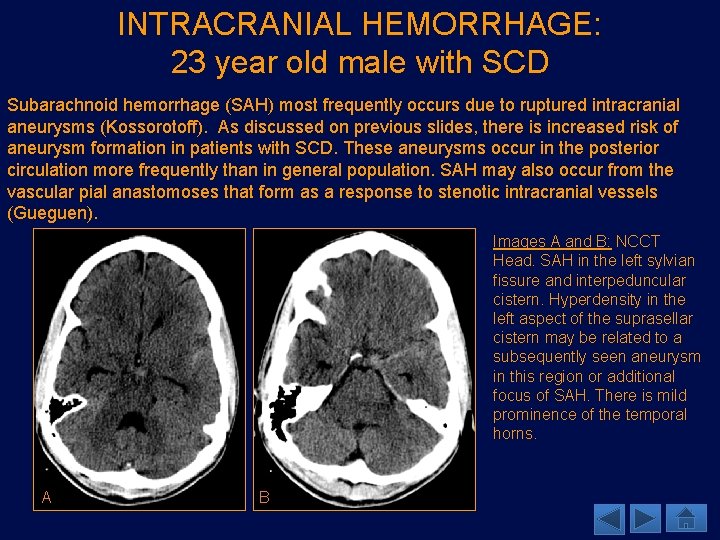
INTRACRANIAL HEMORRHAGE: 23 year old male with SCD Subarachnoid hemorrhage (SAH) most frequently occurs due to ruptured intracranial aneurysms (Kossorotoff). As discussed on previous slides, there is increased risk of aneurysm formation in patients with SCD. These aneurysms occur in the posterior circulation more frequently than in general population. SAH may also occur from the vascular pial anastomoses that form as a response to stenotic intracranial vessels (Gueguen). Images A and B: NCCT Head. SAH in the left sylvian fissure and interpeduncular cistern. Hyperdensity in the left aspect of the suprasellar cistern may be related to a subsequently seen aneurysm in this region or additional focus of SAH. There is mild prominence of the temporal horns. A B

HEAD AND NECK MANIFESTATIONS • The involvement of the maxillofacial bones and inner ear is documented in patients with SCD. • Maxillofacial bones undergo changes specifically related to SCD. • When the inner ear is involved, patients may present with sensorineural hearing loss (SNHL). In the following slides, we will discuss the different manifestations of SCD in head and neck.

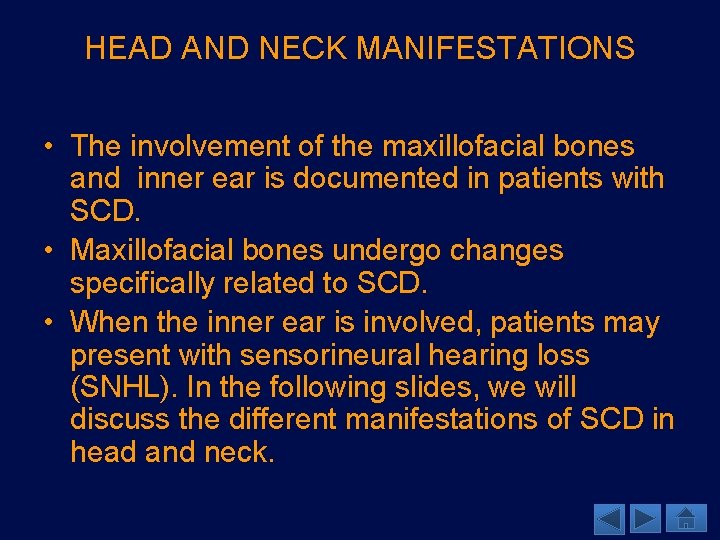
THE MAXILLOFACIAL BONES • Maxillofacial involvement in patients with SCD has been seen as decreased trabeculation, coarse trabecular pattern, and increased spacing in the trabecular pattern. • Increased spacing in the trabecular pattern is not specific to SCD, but is commonly seen with it. • Pathophysiology: • Likely due to marrow expansion from chronic anemia causing thinning of the trabecula. • Marrow expansion causes decreased bone demineralization. • Imaging findings: • The stepladder appearance (A) between mandibular molars is also often found in patients with SCD (Neves) and is secondary to a horizontal trabecular arrangement. A Image A: Sagittal CT of the maxillofacial bones demonstrates a “stepladder” trabeculation pattern (arrows) between roots of the mandibular molars, a finding that can be seen in SCD.

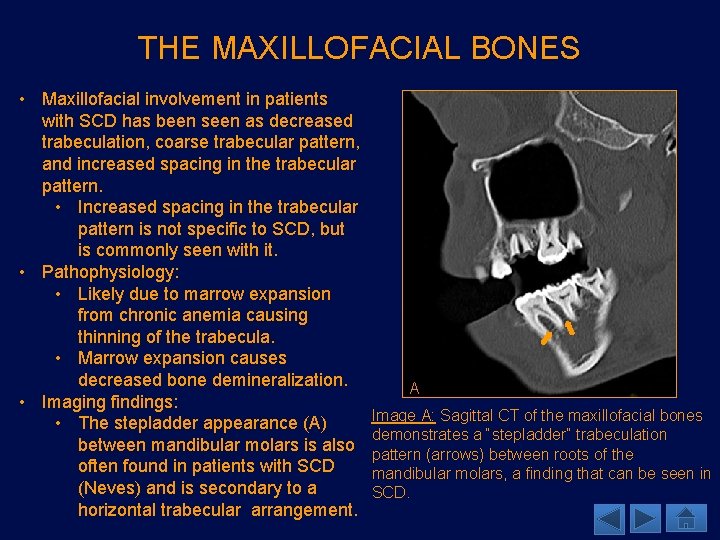
THE MAXILLOFACIAL BONES • Although osteonecrosis of the maxillofacial bones is mostly associated with use of bisphonates, similar findings can be seen in SCD. • Imaging findings: • MRI is better in evaluation of infarcts as it demonstrates areas of high signal on T 2 WIs and STIR images. T 1 hypointensity also seen, frequently with a serpiginous pattern. • Post contrast images show heterogeneous rim-like enhancement (Saito). A • It is important to evaluate for osteomyelitis as areas of infarction Image A: 54 year old male with history of SCD. Sagittal T 1 WI demonstrates predominantly T 1 are susceptible to infection. hypointense signal in the left mandibular condyle (arrow), consistent with osteonecrosis.

THE INNER EAR • • • Labyrinthine hemorrhage (LH) and labyrinthitis ossificans (LO) are associated with SCD and may be complicated by SNHL (Saito). Patients with LH may present with sudden-onset SNHL and vertigo. • LH: Male predominance, more likely to occur in Hb. SC • LO: Male predominance, more likely to occur in Hb. SS Pathophysiology: • Vaso-occlusive complications of the labyrinthine blood vessels is the likely etiology. LH is thought to initiate a reparative response with eventual sclerosis giving rise to LO. Imaging findings: • LH: often in basal turn of the cochlea and vestibule • MR: The T 1 WI demonstrates high intensity in the areas of hemorrhage (but can be variable depending on the stage of blood). • Post contrast images help distinguish LH from local tumors such as schwannomas or hemangiomas. • High resolution T 2 WI are said to show reduced water signal (Saito). • LO is often in the lateral semicircular canals (Saito). • CT: very useful, demonstrates ossification of the membranous labyrinth. • MR: The labyrinth is not visualized on the T 2 WIs. • Post contrast MR images may demonstrate mild enhancement of the labyrinth if there is active labyrinthitis. • Distinguishing between post meningitic LO and LO secondary to SCD is difficult based on imaging, although post meningitic LO has been found to affect the scala tympani of the basal turn of cochlea. Bilateral involvement may be seen in both causes of LO.

SKELETAL MANIFESTATIONS • Skeletal abnormalities due to SCD range from bone infarcts/osteonecrosis to osteomyelitis. • The osseous structures also play an important part in marrow expansion due to the chronically anemic states. In the following slides, we will discuss different skeletal complications patients with SCD present with.

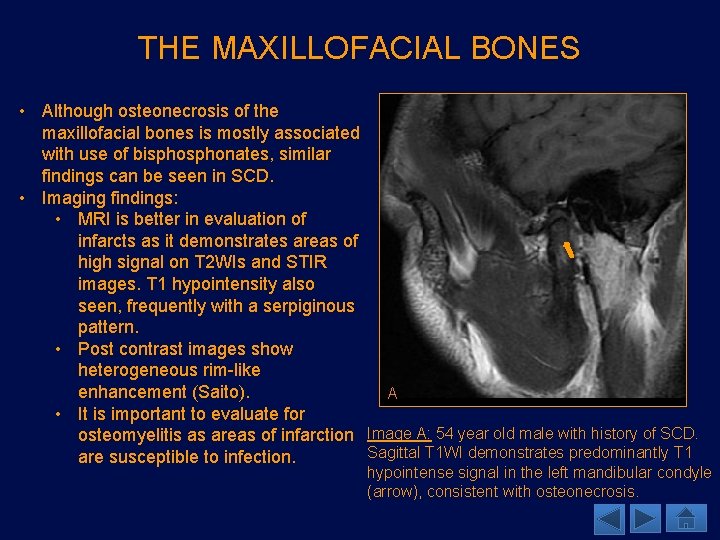
MARROW EXPANSION • Chronic anemia in patients with SCD initiates a restorative response with increased marrow expansion. • Subsequent osteoporosis develops as an increase in hematopoietic marrow leads to trabecular thinning. • Imaging findings: • CT: thinning of the inner and outer table of calvarium, with expansion of the diploic space. • MR: hypointense marrow signal on T 1, compatible with red marrow presence. A 56 year old male with SCD demonstrates calvarial marrow expansion. Sagittal T 1 WI (A) shows diffusely low marrow signal with expansion of the diploic space. Coronal CT (B) demonstrates thinning of the inner and outer tables with expansion of the diploic space. B

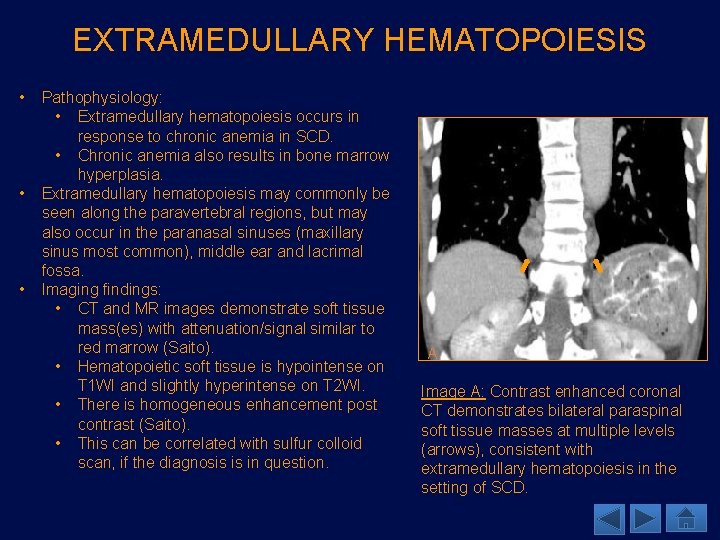
EXTRAMEDULLARY HEMATOPOIESIS • • • Pathophysiology: • Extramedullary hematopoiesis occurs in response to chronic anemia in SCD. • Chronic anemia also results in bone marrow hyperplasia. Extramedullary hematopoiesis may commonly be seen along the paravertebral regions, but may also occur in the paranasal sinuses (maxillary sinus most common), middle ear and lacrimal fossa. Imaging findings: • CT and MR images demonstrate soft tissue mass(es) with attenuation/signal similar to red marrow (Saito). • Hematopoietic soft tissue is hypointense on T 1 WI and slightly hyperintense on T 2 WI. • There is homogeneous enhancement post contrast (Saito). • This can be correlated with sulfur colloid scan, if the diagnosis is in question. A Image A: Contrast enhanced coronal CT demonstrates bilateral paraspinal soft tissue masses at multiple levels (arrows), consistent with extramedullary hematopoiesis in the setting of SCD.

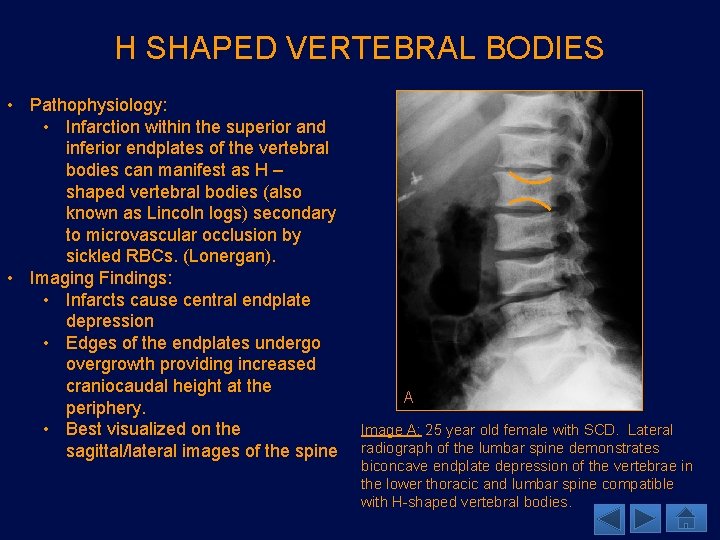
H SHAPED VERTEBRAL BODIES • Pathophysiology: • Infarction within the superior and inferior endplates of the vertebral bodies can manifest as H – shaped vertebral bodies (also known as Lincoln logs) secondary to microvascular occlusion by sickled RBCs. (Lonergan). • Imaging Findings: • Infarcts cause central endplate depression • Edges of the endplates undergo overgrowth providing increased craniocaudal height at the periphery. • Best visualized on the sagittal/lateral images of the spine A Image A: 25 year old female with SCD. Lateral radiograph of the lumbar spine demonstrates biconcave endplate depression of the vertebrae in the lower thoracic and lumbar spine compatible with H-shaped vertebral bodies.

OSTEOMYELITIS AND BONE INFARCTS • Osteomyelitis (OM) and infarction may have similar clinical presentation (pain, fever, erythema and leukocytosis) (Lonergan). Distinguishing between the two may be clinically and radiographically difficult. • There is an increased risk of developing OM in tissues that are infarcted therefore, patients with SCD are more prone to OM than the general population. • Salmonella > Staphlococcus aureus and gram-negative enteric bacilli (Saito). • Imaging Findings: Significant overlap between OM and infarct • XR: periostitis and osteopenia may be seen in OM, but may also be seen in infarctions. • CT: subperiosteal and soft tissue collections, also nonspecific. • MR: Increased marrow signal on T 2 WI and STIR with T 1 hypointensity in areas of OM. Fat saturated post contrast T 1 WI may demonstrate areas of enhancement in OM. This may also be seen in bone infarcts (Lonergan). • T 2 hyperintensity within intervertebral disc and presence of enhancing collections (i. e. epidural abscess) can be helpful distinguishing features.

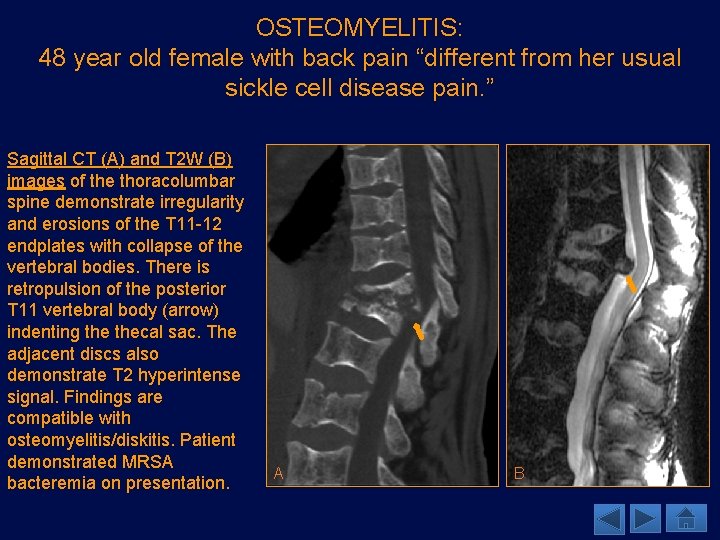
OSTEOMYELITIS: 48 year old female with back pain “different from her usual sickle cell disease pain. ” Sagittal CT (A) and T 2 W (B) images of the thoracolumbar spine demonstrate irregularity and erosions of the T 11 -12 endplates with collapse of the vertebral bodies. There is retropulsion of the posterior T 11 vertebral body (arrow) indenting thecal sac. The adjacent discs also demonstrate T 2 hyperintense signal. Findings are compatible with osteomyelitis/diskitis. Patient demonstrated MRSA bacteremia on presentation. A B

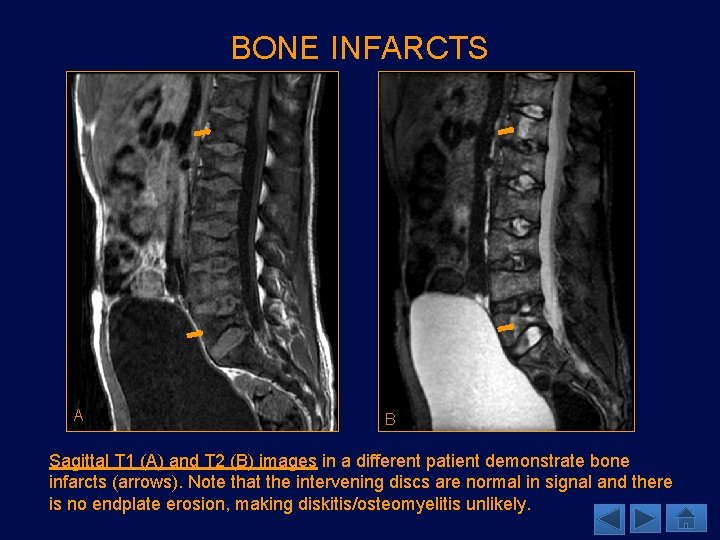
BONE INFARCTS A B Sagittal T 1 (A) and T 2 (B) images in a different patient demonstrate bone infarcts (arrows). Note that the intervening discs are normal in signal and there is no endplate erosion, making diskitis/osteomyelitis unlikely.

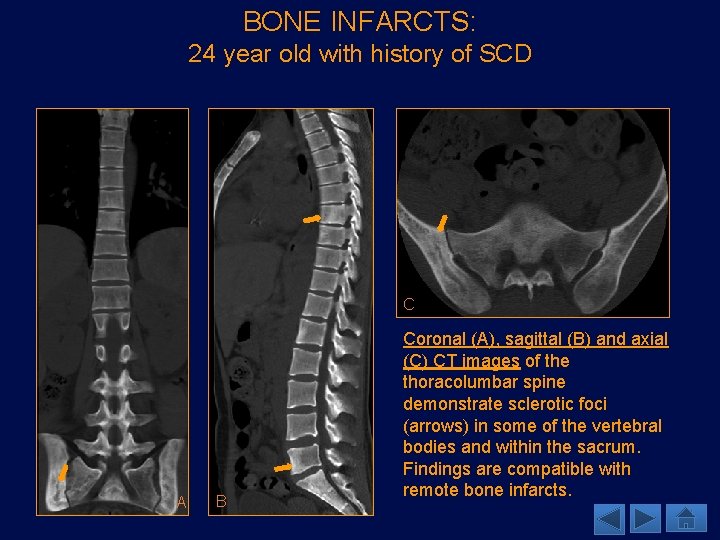
BONE INFARCTS: 24 year old with history of SCD C A B Coronal (A), sagittal (B) and axial (C) CT images of the thoracolumbar spine demonstrate sclerotic foci (arrows) in some of the vertebral bodies and within the sacrum. Findings are compatible with remote bone infarcts.

TREATMENT • Blood exchange transfusion is an important tool in the management of patients with SCD. The Stroke Prevention in Sickle Cell Anemia (STOP) study showed a reduction of stroke risk by 92% with blood exchange transfusion in children with SCD (Gueguen). Patients with high risk of developing strokes are recommended to have maintenance transfusions. Complications of blood transfusions may include transfusion reactions, possibility of acquiring an infection, alloimmunization and iron overload. Patients often require treatment with deferoxamine concurrently (Lonergan). • Bone marrow allograft is considered a curative treatment (Gueguen). The patient’s marrow is repopulated with non-HBSS producing cells. Although this is a restorative process, transplantation may not provide any benefit if the patient has already undergone complications of the disease (bone infarcts, strokes, infections, etc. ) (Lonergan). The evaluation of a patient for bone marrow transplantation do not have any set guidelines currently.

TREATMENT • Hydroxyurea is used to increase formation of Hb. F (fetal hemoglobin), which replaces the abnormal globin chains. Additionally, Hb. F prevents endothelial adhesion of RBCs and increases hemoglobin concentration (Lonergan). • Dura and arachnoid are opened and the superficial temporal artery adventitia is directly sutured to the pia. This results in development of collaterals from the superficial temporal artery to the middle cerebral artery branches. In patients that are considered optimized for preventing non-neurologic sequela of sickle cell disease may still be at increased risk for neurologic complications. In those patients who are at risk for complications related to Moyamoya, pial syangiosis may be an effective treatment in preventing infarcts in children.

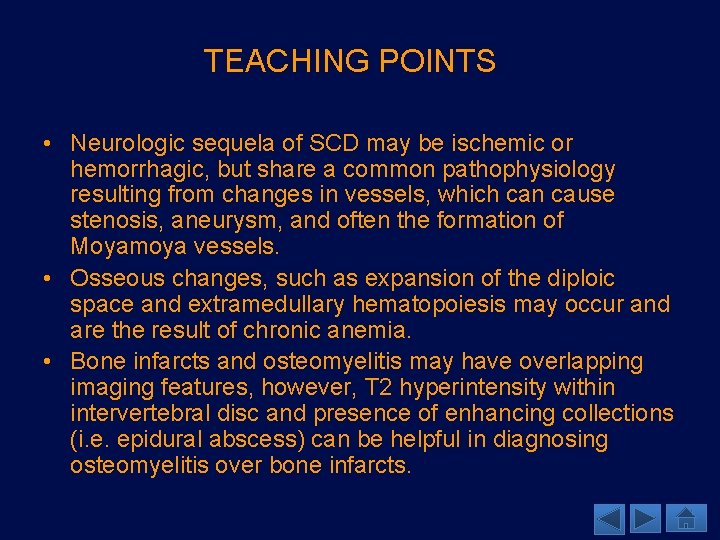
TEACHING POINTS • Neurologic sequela of SCD may be ischemic or hemorrhagic, but share a common pathophysiology resulting from changes in vessels, which can cause stenosis, aneurysm, and often the formation of Moyamoya vessels. • Osseous changes, such as expansion of the diploic space and extramedullary hematopoiesis may occur and are the result of chronic anemia. • Bone infarcts and osteomyelitis may have overlapping imaging features, however, T 2 hyperintensity within intervertebral disc and presence of enhancing collections (i. e. epidural abscess) can be helpful in diagnosing osteomyelitis over bone infarcts.

REFERENCES • • • Gueguen A, Mahevas M, et al. Sickle-cell disease stroke throughout life: A retrospective study in an adult referral center. Am J Hematol. 2014; 89: 267 -272. Lonergan G, Cline D, et al. Sickle Cell Anemia. Radio. Graphics. 2001; 21: 971 -994. Kennedy, B, Mc. Dowell, M, et al. Pial synangiosis for moya syndrome in children with sickle cell anemia: a comprehensive review of reported cases. Neurosurg Focus. 2014 Jan; 36(1): E 12. doi: 10. 3171/2013. Kossorotoff M, Brousse V, et al. Cerebral haemorrhagic risk in children with sickle-cell disease. Published Online First: 30 August 2014. doi: 10. 1111/dmcn. 12571. Moser F, Miller F, et al. The Spectrum of Brain MR Abnormalities in Sickle-Cell Disease: A Report from the Cooperative Study of Sickle Cell Disease. AJNR Am J Neuroradiol 1996; 17: 965 -972. Nabavizadeh SA, Vossough A, Ichord RN, et al. Intracranial aneurysms in sickle cell anemia: clinical and imaging findings. J Neuro. Intervent Surg Published Online First: 18 February 2015. doi: 10. 1136/neuintsurg-2014 -011572. Neves F, Passos C, et al. Correlation between maxillofacial radiographic features and systemic severity as sickle cell disease severity predictor. Clin Oral Invest. 2012; 16: 827 -833. Robertson, R, Burrows, P, et al. Angiographic Changes after Pial Synangiosis in Childhood Moyamoya Disease. AJNR. 1997 May; 18(5): 837 -45. Thust S, Burke C, Siddiqui A. Neuroimaging findings in sickle cell disease. Br J Radiol. 2014; 87: 20130699. Saito N, Nadgir R, et al. Clinical and Radiologic Manifestattions of Sickle Cell Disease in the Head and Neck. Radio. Graphics. 2010; 30: 1021 -1035. Saito N, Wantanabe M, et al. Clinical and Radiologic Findings of Inner Ear Involvement in Sickle Cell Disease. AJNR Am J Neuroradiol. 2011; 32: 2160 -64.
 Gene therapy for sickle cell disease
Gene therapy for sickle cell disease Codominance example
Codominance example Types of sickle cell disease
Types of sickle cell disease Dactylitis
Dactylitis Faranoush
Faranoush Neuroimaging
Neuroimaging Merle sepp
Merle sepp Genotypic ratio of monohybrid cross
Genotypic ratio of monohybrid cross Chapter 14 the human genome making karyotypes answer key
Chapter 14 the human genome making karyotypes answer key Life expectancy of sickle cell patients
Life expectancy of sickle cell patients Anemia structure
Anemia structure Difference between sickle cell anaemia and thalassemia
Difference between sickle cell anaemia and thalassemia Aplastic crisis
Aplastic crisis Cri du chat chromosomal abnormalities
Cri du chat chromosomal abnormalities 4 steps of protein synthesis
4 steps of protein synthesis Pethidine in sickle cell
Pethidine in sickle cell Hemophilia karyotype picture
Hemophilia karyotype picture Pedigree key
Pedigree key Sickle cell hemoglobin structure
Sickle cell hemoglobin structure Sickle cell punnett square
Sickle cell punnett square Is sickle cell anemia codominant
Is sickle cell anemia codominant Sickle cell osteomyelitis
Sickle cell osteomyelitis Film
Film Sickle blood cell
Sickle blood cell Sickle cell anemia
Sickle cell anemia Sickle cell codominance
Sickle cell codominance Old world flycatchers
Old world flycatchers Nata sickle cell
Nata sickle cell Sickle cell anemia
Sickle cell anemia Sickle cell pain
Sickle cell pain Hemoglobin electrophoresis
Hemoglobin electrophoresis Anemia
Anemia Sickle cell anemia due to
Sickle cell anemia due to Inheritance pattern of sickle cell anemia
Inheritance pattern of sickle cell anemia Sickle cell anemia dna sequence
Sickle cell anemia dna sequence Persilangan thalasemia
Persilangan thalasemia Pbs sickle cell
Pbs sickle cell Biomedical waste management definition
Biomedical waste management definition