Lewis Dot Structures E dot diagrams the Octet

- Slides: 13

Lewis Dot Structures E – dot diagrams, the Octet Rule

• The total number of electrons represented in a Lewis structure is equal to the sum of the numbers of valence electrons on each individual atom. • Non-valence electrons are not represented in Lewis structures. • Lewis structures show each atom and its position in the structure of the molecule using its chemical symbol. Pairs of dots or lines are drawn between atoms that are bonded to one another. • Excess electrons that form lone pairs are represented as pairs of dots, and are placed next to the atoms. These are unbonded pairs.


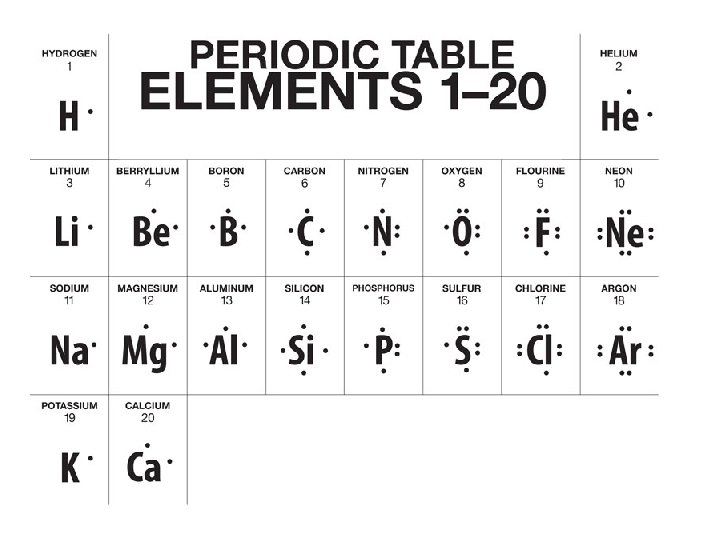
Octet Rule • The octet rule states that atoms of low (<20) atomic number tend to combine in such a way that they each have eight electrons in their valence shells, giving them the same electronic configuration as a noble gas. • The rule is applicable to the main-group elements, especially carbon, nitrogen, oxygen, and the halogens, but also to metals such as sodium or magnesium. • Hydrogen and helium are exceptions; they want to have two electrons (a duet) in their valence shells.

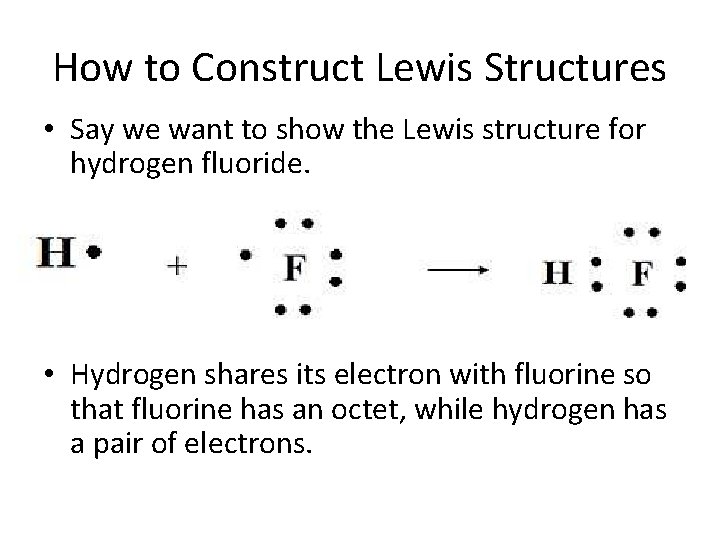
How to Construct Lewis Structures • Say we want to show the Lewis structure for hydrogen fluoride. • Hydrogen shares its electron with fluorine so that fluorine has an octet, while hydrogen has a pair of electrons.

Steps for Lewis Structures 1. Count the total number of valence electrons. 2. Decide on the central atom. It is usually the atom with the lowest subscript in the formula and it is the atom which can form the most bonds. 3. Arrange electrons around the atoms so that each atom has an octet (or two if hydrogen) if possible. 4. Electrons should be placed initially as lone pairs: one pair of dots for each pair of electrons available. Lone pairs should initially be placed on outer atoms (other than hydrogen) until each outer atom has eight electrons in bonding pairs and lone pairs; extra lone pairs may then be placed on the central atom.

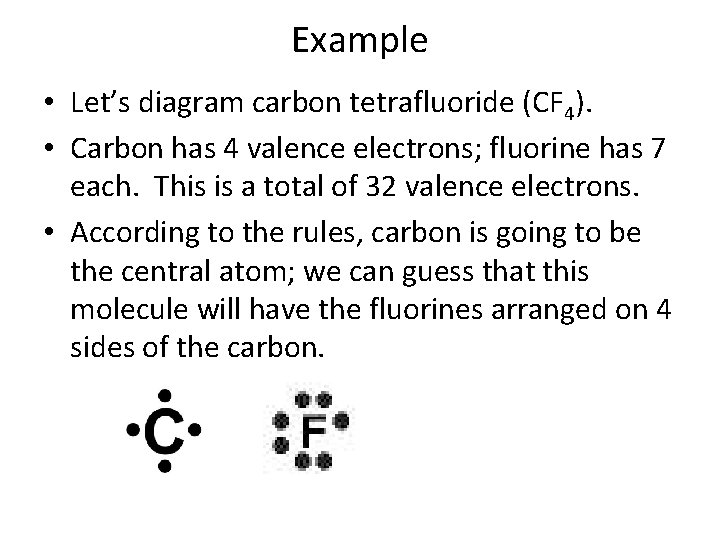
Example • Let’s diagram carbon tetrafluoride (CF 4). • Carbon has 4 valence electrons; fluorine has 7 each. This is a total of 32 valence electrons. • According to the rules, carbon is going to be the central atom; we can guess that this molecule will have the fluorines arranged on 4 sides of the carbon.

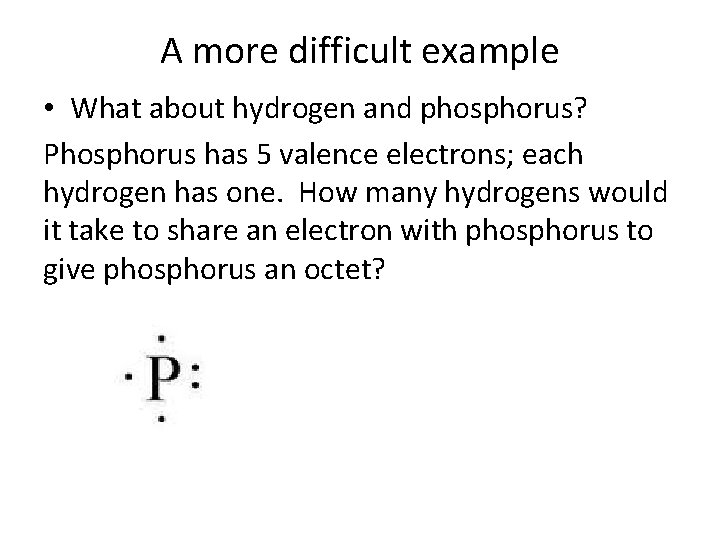
A more difficult example • What about hydrogen and phosphorus? Phosphorus has 5 valence electrons; each hydrogen has one. How many hydrogens would it take to share an electron with phosphorus to give phosphorus an octet?

Steps for Lewis Structures 5. Once all lone pairs are placed, atoms - especially the central atoms - may not have an octet of electrons in pairs. In this case, the atoms must form a double bond. Another way to form a double bond is moving a lone pair of electrons to form a second between the two atoms. As the bonding pair is shared between the two atoms, the atom that originally had the lone pair still has an octet; the other atom now has two more electrons in its valence shell.

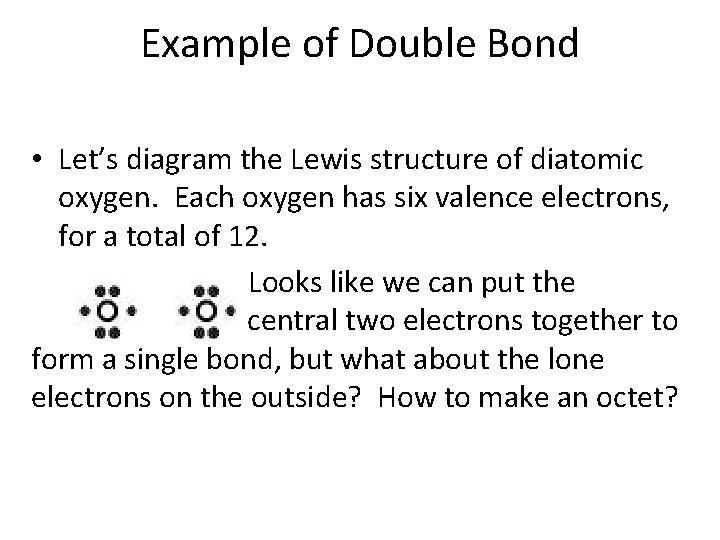
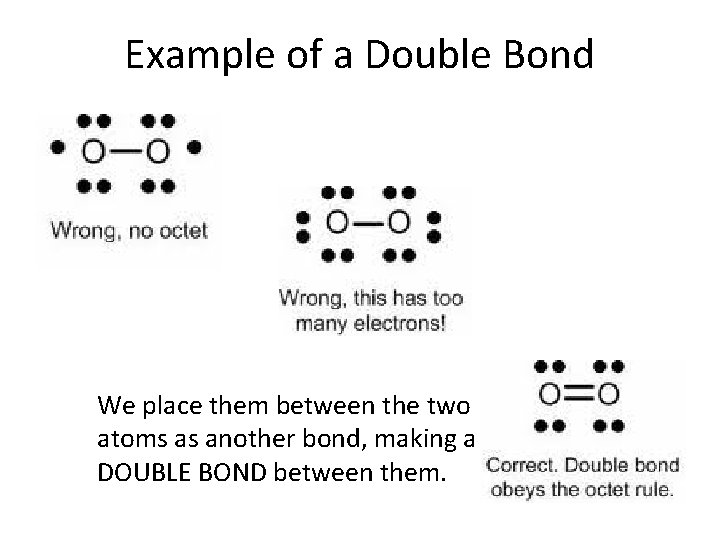
Example of Double Bond • Let’s diagram the Lewis structure of diatomic oxygen. Each oxygen has six valence electrons, for a total of 12. Looks like we can put the central two electrons together to form a single bond, but what about the lone electrons on the outside? How to make an octet?

Example of a Double Bond We place them between the two atoms as another bond, making a DOUBLE BOND between them.

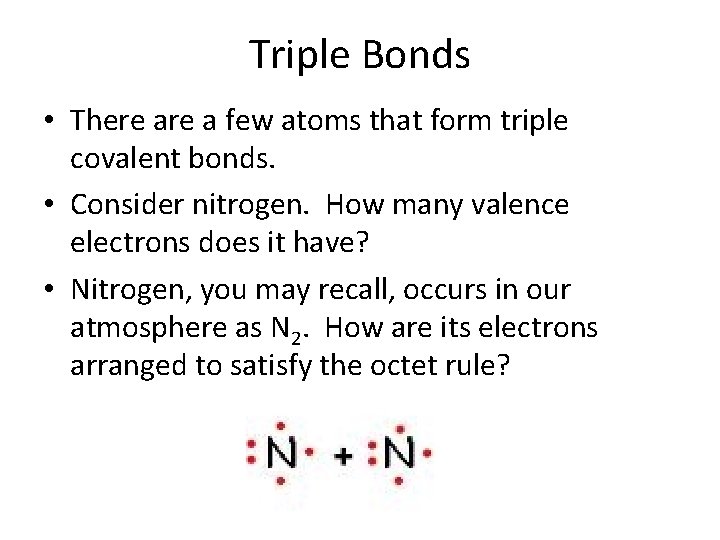
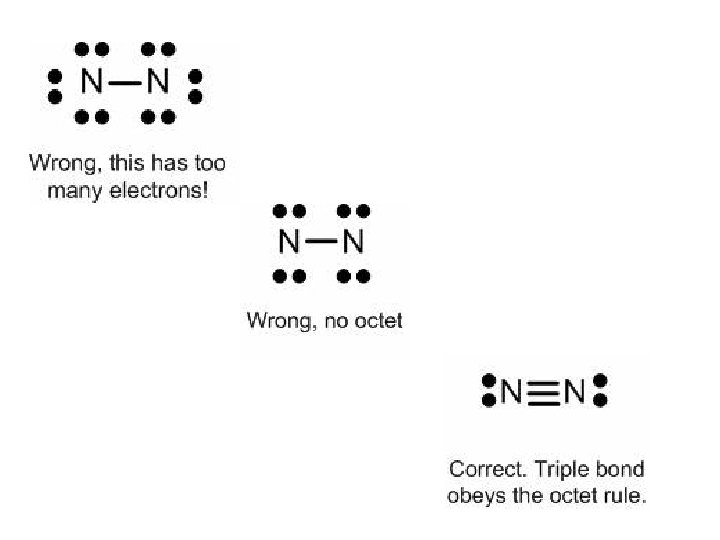
Triple Bonds • There a few atoms that form triple covalent bonds. • Consider nitrogen. How many valence electrons does it have? • Nitrogen, you may recall, occurs in our atmosphere as N 2. How are its electrons arranged to satisfy the octet rule?
