Lesson Overview Properties of Water Lesson Overview 2





- Slides: 14

Lesson Overview Properties of Water Lesson Overview 2. 2 Properties of Water

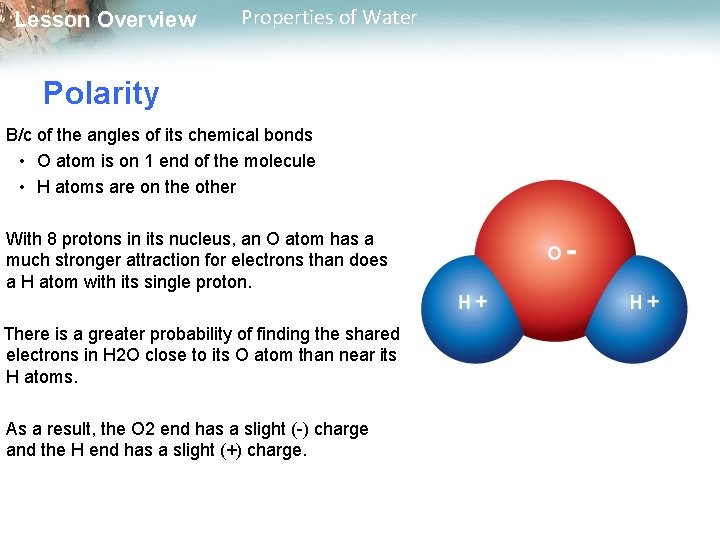
Lesson Overview Properties of Water Polarity B/c of the angles of its chemical bonds • O atom is on 1 end of the molecule • H atoms are on the other With 8 protons in its nucleus, an O atom has a much stronger attraction for electrons than does a H atom with its single proton. There is a greater probability of finding the shared electrons in H 2 O close to its O atom than near its H atoms. As a result, the O 2 end has a slight (-) charge and the H end has a slight (+) charge.

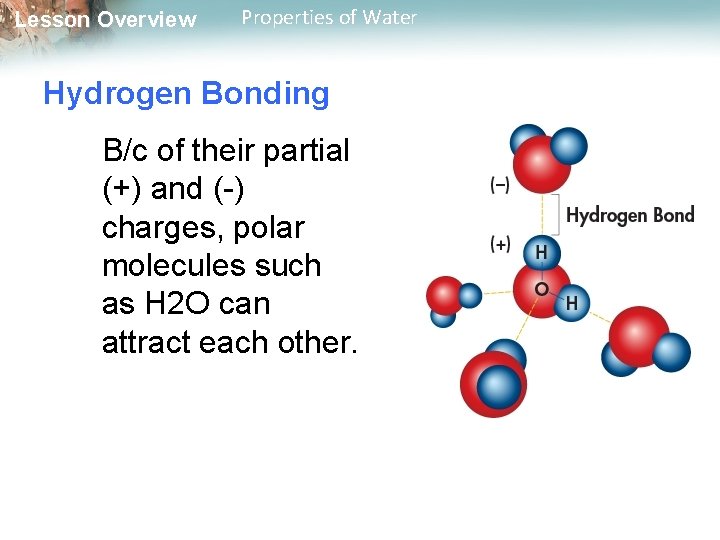
Lesson Overview Properties of Water Hydrogen Bonding B/c of their partial (+) and (-) charges, polar molecules such as H 2 O can attract each other.

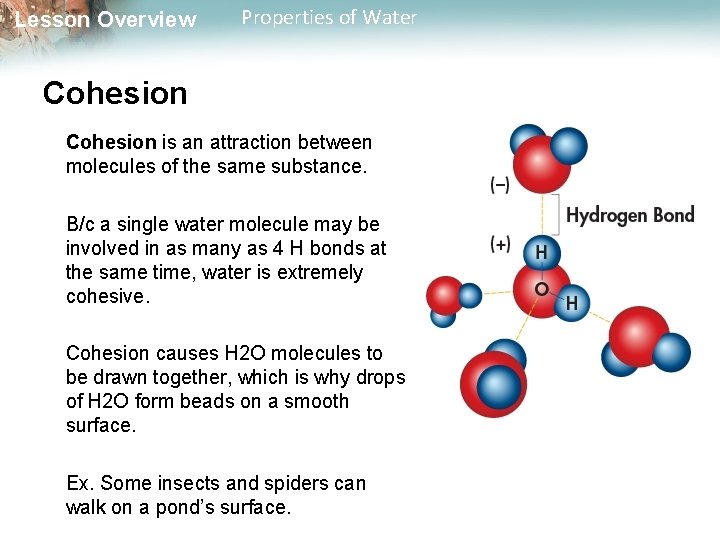
Lesson Overview Properties of Water Cohesion is an attraction between molecules of the same substance. B/c a single water molecule may be involved in as many as 4 H bonds at the same time, water is extremely cohesive. Cohesion causes H 2 O molecules to be drawn together, which is why drops of H 2 O form beads on a smooth surface. Ex. Some insects and spiders can walk on a pond’s surface.

Lesson Overview Properties of Water Adhesion is an attraction between molecules of different substances. The surface of H 2 O in a graduated cylinder dips slightly in the center, forming a curve called a meniscus, • Adhesion between H 2 O molecules and glass molecules is stronger than the cohesion between H 20 molecules. • Adhesion between water and glass also causes water to rise in a narrow tube against the force of gravity. This effect is called capillary action.

Lesson Overview Properties of Water Solutions and Suspensions Water is not always pure; it is often found as part of a mixture. A mixture is a material composed of two or more elements or compounds that are physically mixed together but not chemically combined. Living things are in part composed of mixtures involving water. Two types of mixtures that can be made with water are solutions and suspensions.

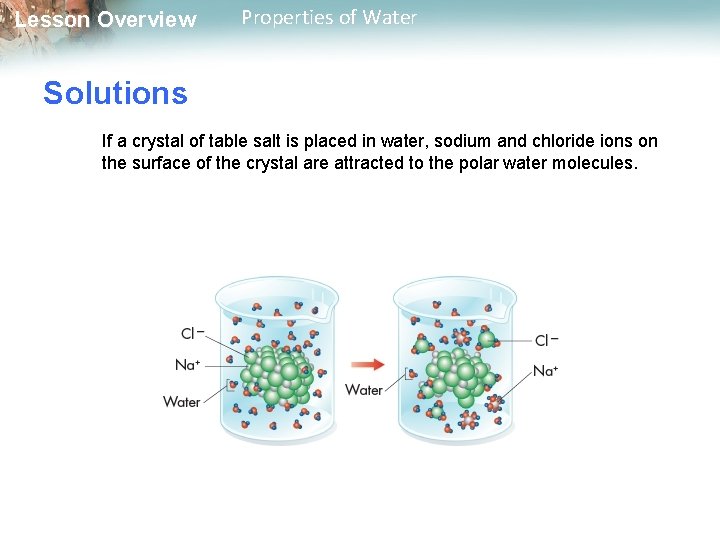
Lesson Overview Properties of Water Solutions If a crystal of table salt is placed in water, sodium and chloride ions on the surface of the crystal are attracted to the polar water molecules.

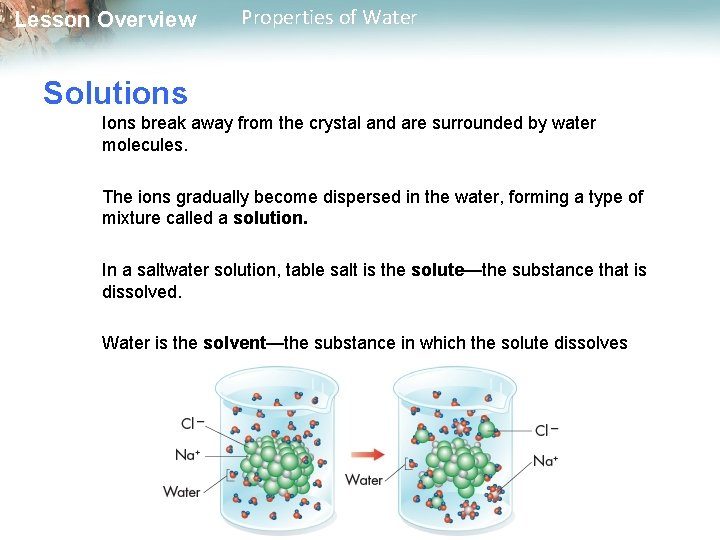
Lesson Overview Properties of Water Solutions Ions break away from the crystal and are surrounded by water molecules. The ions gradually become dispersed in the water, forming a type of mixture called a solution. In a saltwater solution, table salt is the solute—the substance that is dissolved. Water is the solvent—the substance in which the solute dissolves

Lesson Overview Properties of Water Suspensions Some materials do not dissolve when placed in water, but separate into pieces so small that they do not settle out. Such mixtures of water and nondissolved material are known as suspensions. Some of the most important biological fluids are both solutions and suspensions. Blood is mostly water. It contains many dissolved compounds, but also cells and other undissolved particles that remain in suspension as the blood moves through the body.

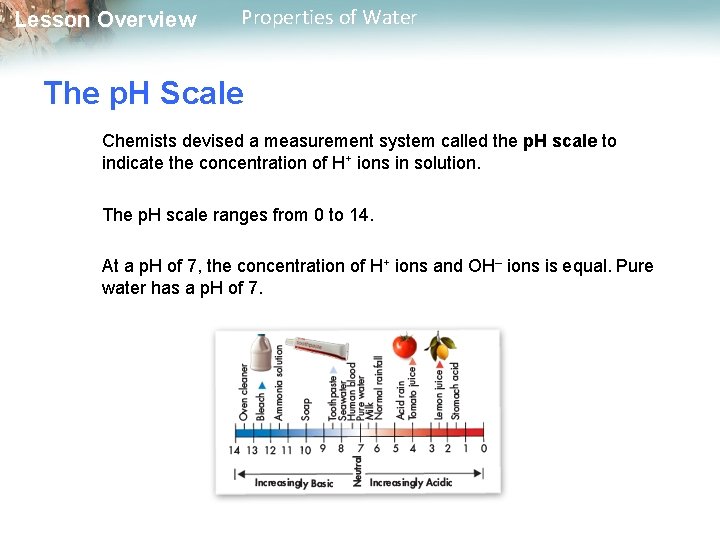
Lesson Overview Properties of Water The p. H Scale Chemists devised a measurement system called the p. H scale to indicate the concentration of H+ ions in solution. The p. H scale ranges from 0 to 14. At a p. H of 7, the concentration of H+ ions and OH– ions is equal. Pure water has a p. H of 7.

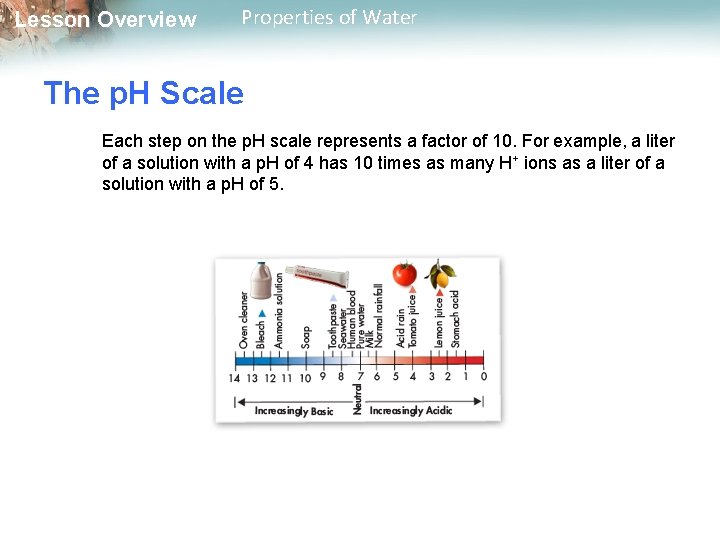
Lesson Overview Properties of Water The p. H Scale Each step on the p. H scale represents a factor of 10. For example, a liter of a solution with a p. H of 4 has 10 times as many H+ ions as a liter of a solution with a p. H of 5.

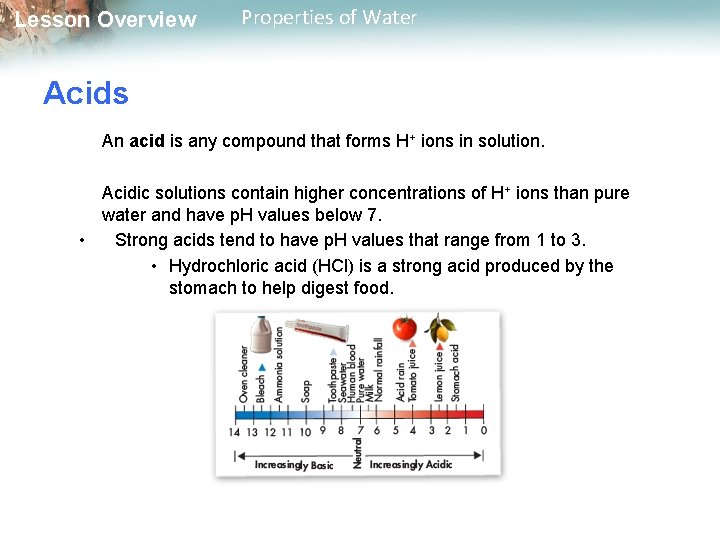
Lesson Overview Properties of Water Acids An acid is any compound that forms H+ ions in solution. Acidic solutions contain higher concentrations of H+ ions than pure water and have p. H values below 7. • Strong acids tend to have p. H values that range from 1 to 3. • Hydrochloric acid (HCl) is a strong acid produced by the stomach to help digest food.

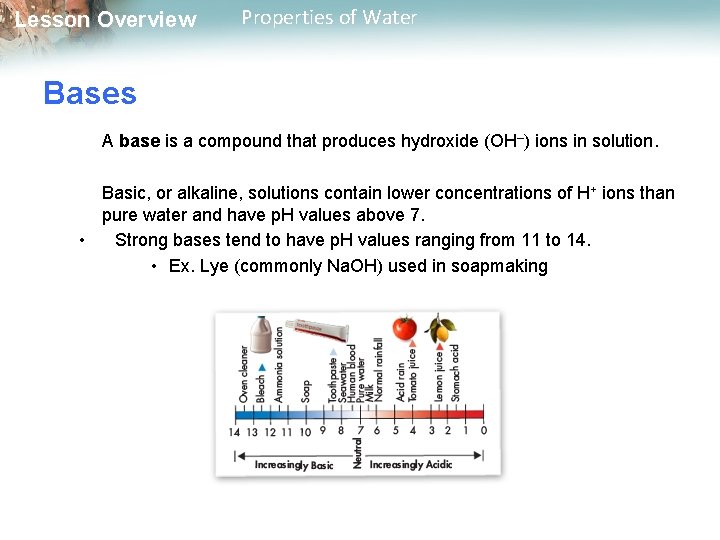
Lesson Overview Properties of Water Bases A base is a compound that produces hydroxide (OH–) ions in solution. Basic, or alkaline, solutions contain lower concentrations of H+ ions than pure water and have p. H values above 7. • Strong bases tend to have p. H values ranging from 11 to 14. • Ex. Lye (commonly Na. OH) used in soapmaking

Lesson Overview Properties of Water Buffers The p. H of the fluids within most cells in the human body must generally be kept between 6. 5 and 7. 5 in order to maintain homeostasis. Organisms control p. H is through dissolved compounds called buffers • Weak acids or bases that can react with strong acids or bases to prevent sharp, sudden changes in p. H.
 Water and water and water water
Water and water and water water Chapter 17 elements and their properties answer key
Chapter 17 elements and their properties answer key Elements and their properties section 1 metals
Elements and their properties section 1 metals Lesson outline physical properties lesson 2
Lesson outline physical properties lesson 2 Lesson outline lesson 2 wave properties answer key
Lesson outline lesson 2 wave properties answer key Extensive vs intensive quantity
Extensive vs intensive quantity Chemical property definition
Chemical property definition Chapter 9 lesson 2 photosynthesis an overview
Chapter 9 lesson 2 photosynthesis an overview Lesson overview
Lesson overview Water properties concept map
Water properties concept map Properties of water ap bio
Properties of water ap bio Characteristics of pure water
Characteristics of pure water Properties of water clipart
Properties of water clipart Properties of water lab
Properties of water lab Ocean water properties
Ocean water properties