Lesson Overview Properties of Water Lesson Overview 2










- Slides: 20

Lesson Overview Properties of Water Lesson Overview 2. 2 Properties of Water Objectives: How does the structure of water contribute to its unique properties?

Lesson Overview Properties of Water THINK ABOUT IT Looking back at Earth from space, an astronaut called it “the blue planet, ” referring to the oceans of water that cover nearly three fourths of Earth’s surface. The very presence of liquid water tells a scientist that life may also be present on such a planet. Why should life itself be connected so strongly to something so ordinary that we often take it for granted? There is something very special about water and the role it plays in living things.

Lesson Overview Properties of Water The Water Molecule How does the structure of water contribute to its unique properties? Because water is a polar molecule, it is able to form multiple hydrogen bonds, which account for many of water’s special properties.

Lesson Overview Properties of Water The Water Molecule Water is one of the few compounds found in a liquid state over most of Earth’s surface. Like other molecules, water (H 2 O) is neutral. The positive charges on its 10 protons balance out the negative charges on its 10 electrons.

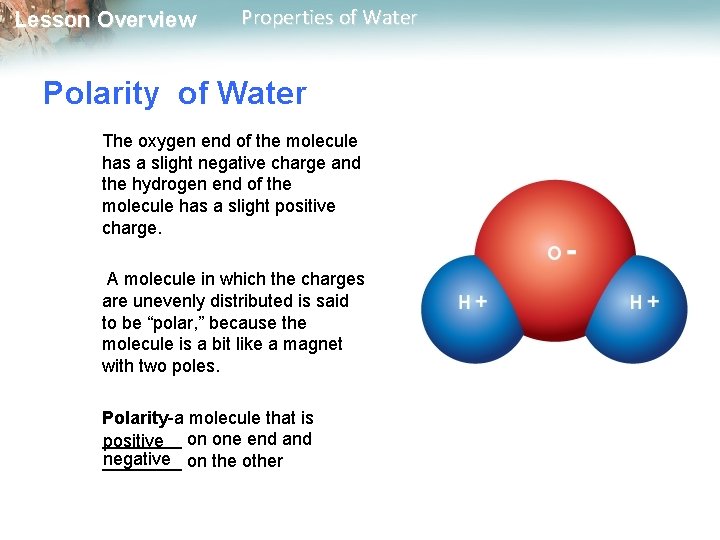
Lesson Overview Properties of Water Polarity of Water The oxygen end of the molecule has a slight negative charge and the hydrogen end of the molecule has a slight positive charge. A molecule in which the charges are unevenly distributed is said to be “polar, ” because the molecule is a bit like a magnet with two poles. Polarity-a molecule that is ____ positive on one end and negative on the other ____

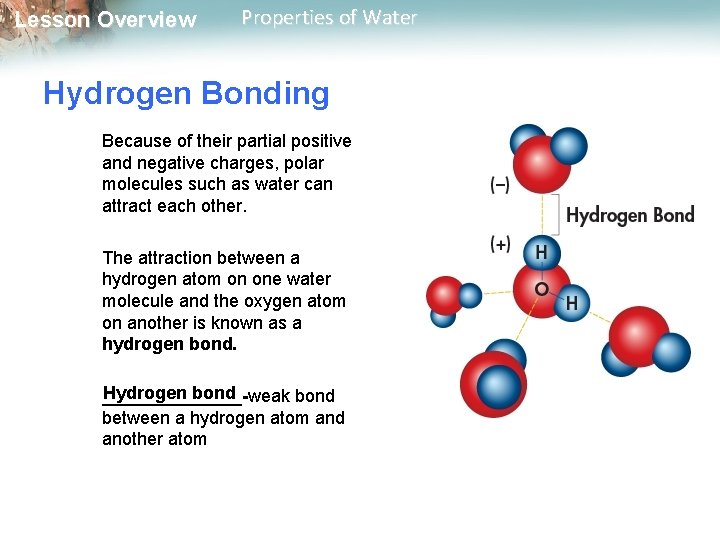
Lesson Overview Properties of Water Hydrogen Bonding Because of their partial positive and negative charges, polar molecules such as water can attract each other. The attraction between a hydrogen atom on one water molecule and the oxygen atom on another is known as a hydrogen bond. Hydrogen bond _______-weak bond between a hydrogen atom and another atom

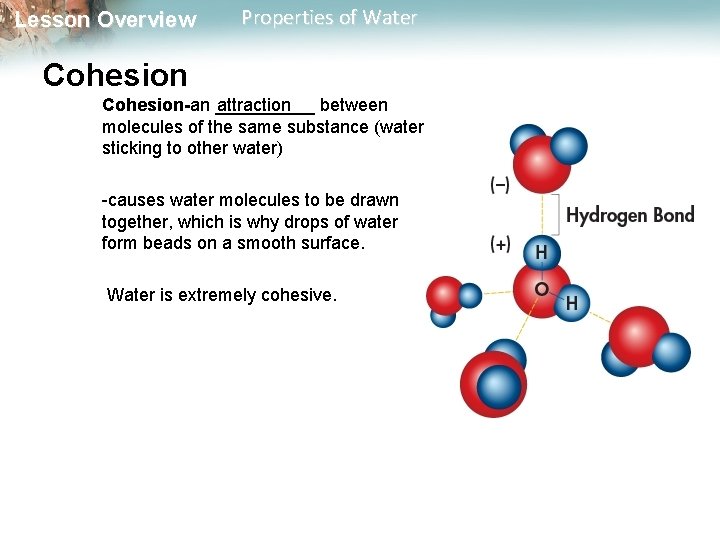
Lesson Overview Properties of Water Cohesion attraction Cohesion-an _____ between molecules of the same substance (water sticking to other water) -causes water molecules to be drawn together, which is why drops of water form beads on a smooth surface. Water is extremely cohesive.

Lesson Overview Properties of Water Surface Tension -this is why drops of water form beads on a smooth surface Surface tension thin “film” on top of water caused by polarity ________-a - Cohesion produces_______, surface tension explaining _________________________ why some insects and spiders can walk on a pond’s surface

Lesson Overview Properties of Water Adhesion-an attraction between molecules of different substances (water sticking to other things) Adhesion between water and glass causes water to rise in a narrow tube against the force of gravity. This effect is called capillary action.

Lesson Overview Properties of Water Adhesion Capillary action-water moving up _____ thin tubes **Capillary action is one of the forces that draws water out of the ______________ roots of a plant and up into its ______________ stems and leaves. Cohesion holds the column of water together as it rises.

Lesson Overview Properties of Water Heat Capacity Water takes a long time to _______ heat up and _____. cool down Large bodies of water, such as oceans and lakes, can absorb large amounts of heat with only small changes in temperature. This protects organisms living within from drastic changes in temperature. water This helps organisms Living organisms are made up of mostly _____. temperature change. resist ______

Lesson Overview Properties of Water Solutions ____-an evenly distributed mixture (salt dissolved in water) Solution Solute-is dissolved (_____) salt Solvent _______-the solute is dissolved in (water) Water dissolves many things!! Water’s polarity gives it the ability to dissolve both ionic compounds and other polar molecules. Water easily dissolves salts, sugars, minerals, gases, and even other solvents such as alcohol.

Lesson Overview Properties of Water Suspensions-mixtures of water and ______ nondissolved material (sand in water). **Some of the most important biological fluids are both solutions and suspensions. Blood is mostly water. It contains many dissolved compounds, but also cells and other undissolved particles that remain in suspension as the blood moves through the body.

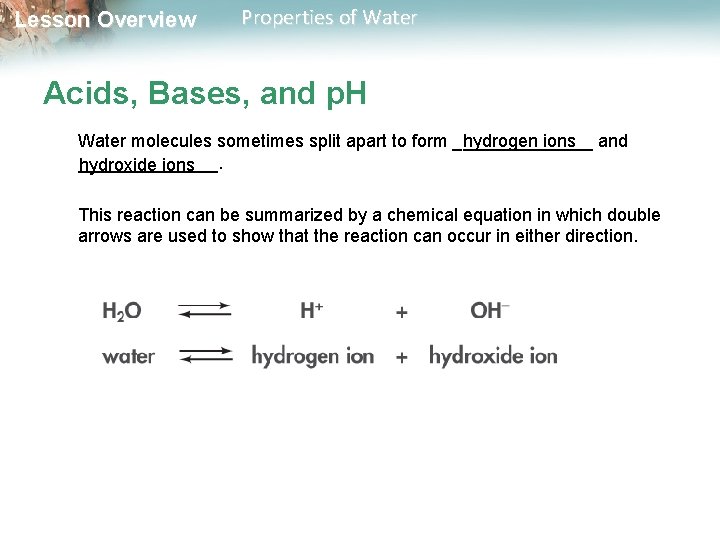
Lesson Overview Properties of Water Acids, Bases, and p. H Water molecules sometimes split apart to form _______ hydrogen ions and _______. hydroxide ions This reaction can be summarized by a chemical equation in which double arrows are used to show that the reaction can occur in either direction.

Lesson Overview Properties of Water Acids, Bases, and p. H In pure water, about 1 water molecule in 550 million splits to form ions in this way. Because the number of positive hydrogen ions produced is equal to the number of negative hydroxide ions produced, pure water is neutral.

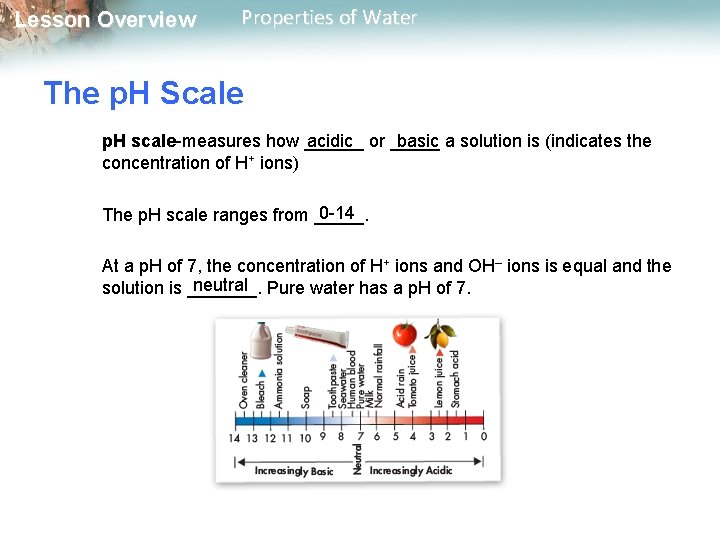
Lesson Overview Properties of Water The p. H Scale p. H scale-measures how ______ acidic or _____ basic a solution is (indicates the concentration of H+ ions) 0 -14 The p. H scale ranges from _____. At a p. H of 7, the concentration of H+ ions and OH– ions is equal and the neutral Pure water has a p. H of 7. solution is _______.

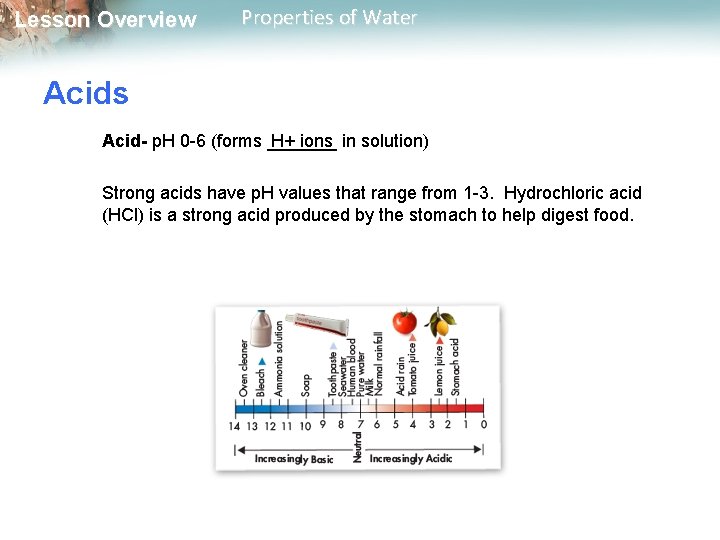
Lesson Overview Properties of Water Acids Acid- p. H 0 -6 (forms _______ H+ ions in solution) Strong acids have p. H values that range from 1 -3. Hydrochloric acid (HCl) is a strong acid produced by the stomach to help digest food.

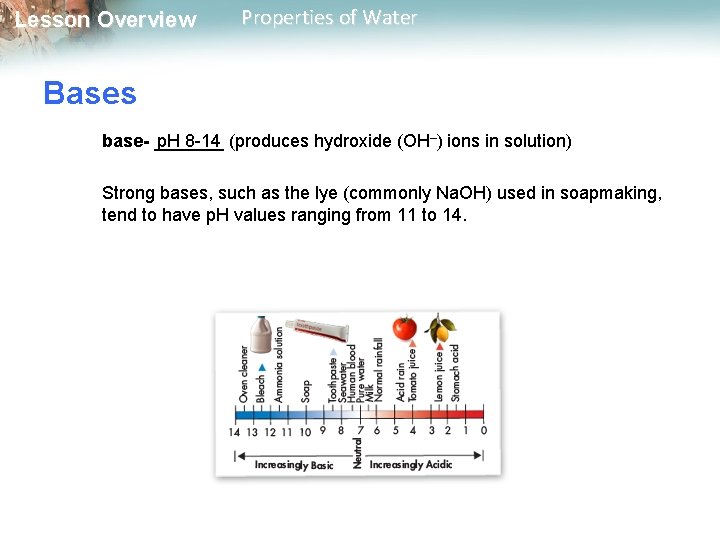
Lesson Overview Properties of Water Bases base- _______ p. H 8 -14 (produces hydroxide (OH–) ions in solution) Strong bases, such as the lye (commonly Na. OH) used in soapmaking, tend to have p. H values ranging from 11 to 14.

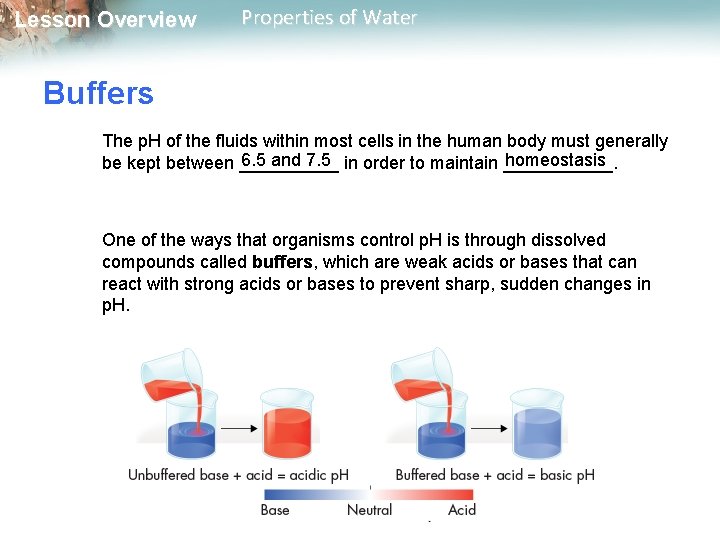
Lesson Overview Properties of Water Buffers The p. H of the fluids within most cells in the human body must generally 6. 5 and 7. 5 in order to maintain ______. homeostasis be kept between _____ One of the ways that organisms control p. H is through dissolved compounds called buffers, which are weak acids or bases that can react with strong acids or bases to prevent sharp, sudden changes in p. H.

Lesson Overview Properties of Water Buffers Adding acid to an unbuffered solution causes the p. H of the unbuffered solution to drop. If the solution contains a buffer, however, adding the acid will cause only a slight change in p. H. **Acids and basis are very important in living organisms -they affect the rate of chemical reactions.
 Water and water and water water
Water and water and water water Carbon family
Carbon family Chapter 17 overview elements and their properties
Chapter 17 overview elements and their properties Lesson outline lesson 2 - physical properties answer key
Lesson outline lesson 2 - physical properties answer key Lesson outline lesson 2 wave properties answer key
Lesson outline lesson 2 wave properties answer key Intensive and extensive properties
Intensive and extensive properties Physical properties of ice cube
Physical properties of ice cube Chapter 9 lesson 2 photosynthesis an overview
Chapter 9 lesson 2 photosynthesis an overview Lesson overview
Lesson overview Concept map on water
Concept map on water Properties of water ap biology
Properties of water ap biology Physical properties of sea water
Physical properties of sea water Properties of water clipart
Properties of water clipart Properties of water lab
Properties of water lab Ocean water properties
Ocean water properties Properties of water in matter
Properties of water in matter Bill nye properties of water
Bill nye properties of water Honors biology properties of water lab
Honors biology properties of water lab The extraordinary properties of water
The extraordinary properties of water The extraordinary properties of water
The extraordinary properties of water Properties of water foldable
Properties of water foldable