Lecture 6 Determination of arsenic and antimony A





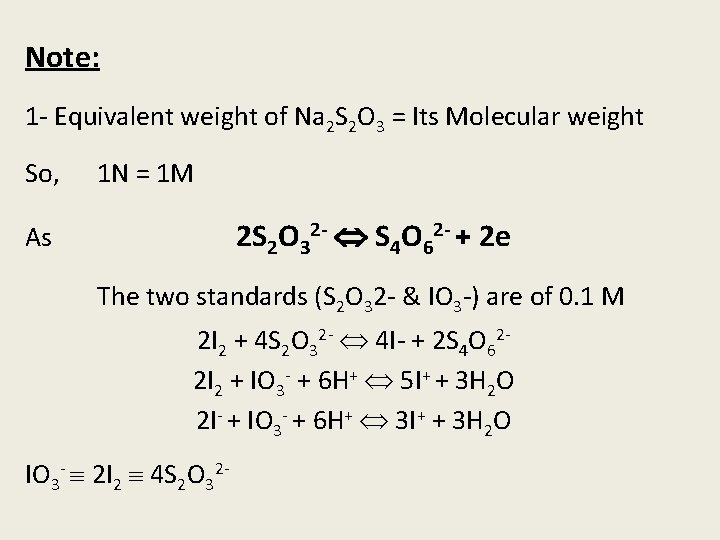




- Slides: 18

Lecture 6

Determination of arsenic and antimony A. Determination of trivalent arsenic and antimony (AS 2 O 3 &Sb 2 O 3) Ø Trivalent arsenic and antimony are reducing agents Ø They can be detected by oxidizing agents A. 1. Iodine A. 2. Iodate A. 3. Bromate

A. 1. With iodine Trivalent arsenic and antimony are oxidized with iodine in neutral medium (p. H 6. 5) to pentavalent state. AS 2 O 3 +2 I 2 + 2 H 2 O AS 2 O 5 + 4 HI Arsenic trioxide Arsenic pentaoxide Sb 2 O 3 + 2 I 2 + 2 H 2 O Sb 2 O 5 + 4 HI This reaction is reversible. So we must remove HI by addition of Na. HCO 3. Na 2 CO 3 and Na. OH cannot used because they react with I 2 and form iodide, hypoiodite and iodate.

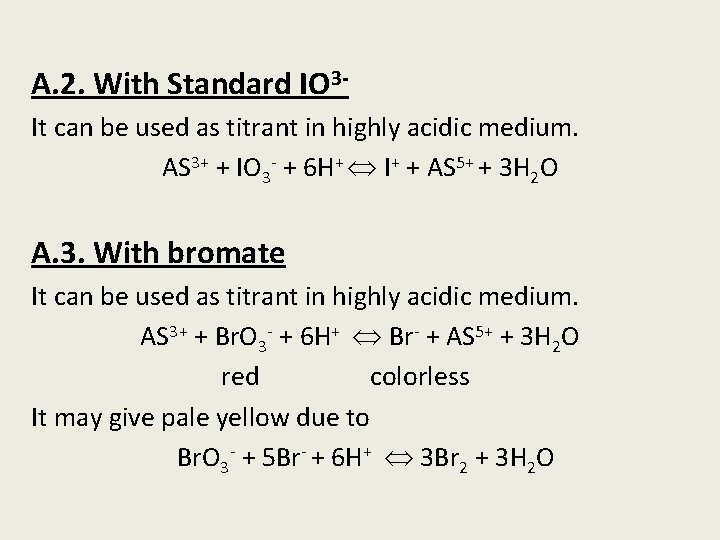
A. 2. With Standard IO 3 It can be used as titrant in highly acidic medium. AS 3+ + IO 3 - + 6 H+ I+ + AS 5+ + 3 H 2 O A. 3. With bromate It can be used as titrant in highly acidic medium. AS 3+ + Br. O 3 - + 6 H+ Br- + AS 5+ + 3 H 2 O red colorless It may give pale yellow due to Br. O 3 - + 5 Br- + 6 H+ 3 Br 2 + 3 H 2 O

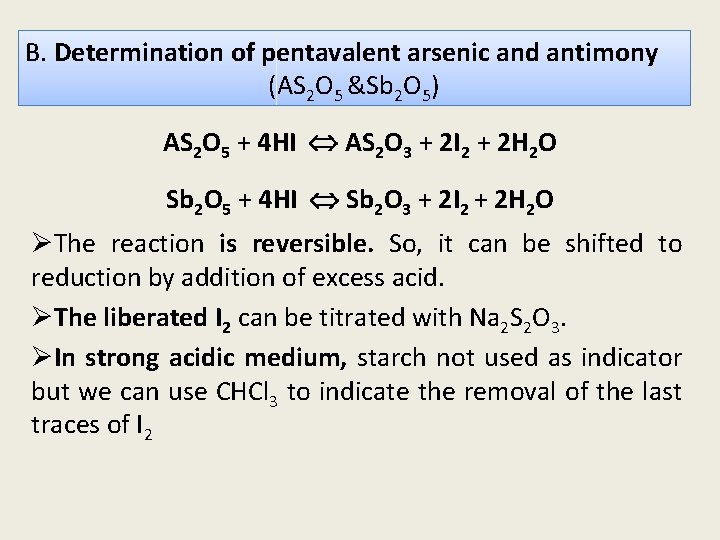
B. Determination of pentavalent arsenic and antimony (AS 2 O 5 &Sb 2 O 5) AS 2 O 5 + 4 HI AS 2 O 3 + 2 I 2 + 2 H 2 O Sb 2 O 5 + 4 HI Sb 2 O 3 + 2 I 2 + 2 H 2 O ØThe reaction is reversible. So, it can be shifted to reduction by addition of excess acid. ØThe liberated I 2 can be titrated with Na 2 S 2 O 3. ØIn strong acidic medium, starch not used as indicator but we can use CHCl 3 to indicate the removal of the last traces of I 2

Note: for titration involving iodine ØIn presence of alcohol or conc acids, organic solvents are recommended as indicators. Not starch…. ØI 2 is soluble in CHCl 3 or CCl 4 90 times more than in H 2 O give violet colour and then become colorless at the end point. ØIt is important that the mixture be shaken well near the end point to enable aqueous sodium thiosulfate to react with the last traces of iodine in the organic phase.

Determination of Sn 2+ and Sn 4+ Sn 2+ Sn 4+ + 2 e ØStannous determined iodimetrically (titration with iodine). ØStannic can be determined by first reduction to stannous by treatment with metalic reducing agent such as zinc, then titrate the resultant Sn 2+

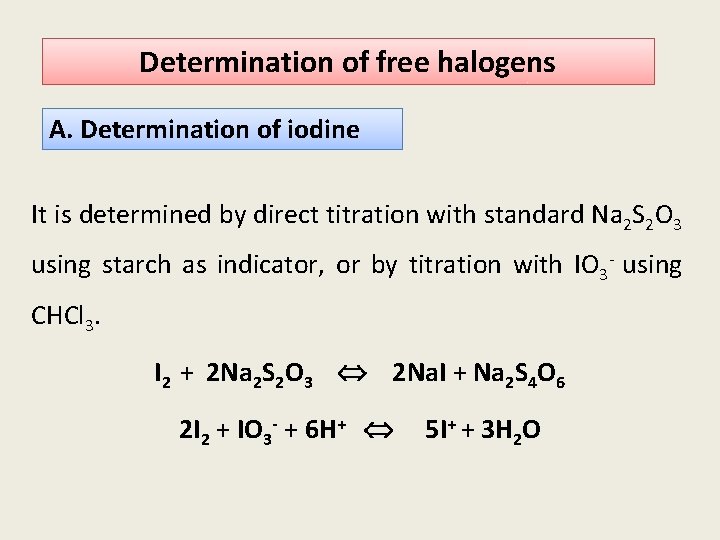
Determination of free halogens A. Determination of iodine It is determined by direct titration with standard Na 2 S 2 O 3 using starch as indicator, or by titration with IO 3 - using CHCl 3. I 2 + 2 Na 2 S 2 O 3 2 Na. I + Na 2 S 4 O 6 2 I 2 + IO 3 - + 6 H+ 5 I+ + 3 H 2 O

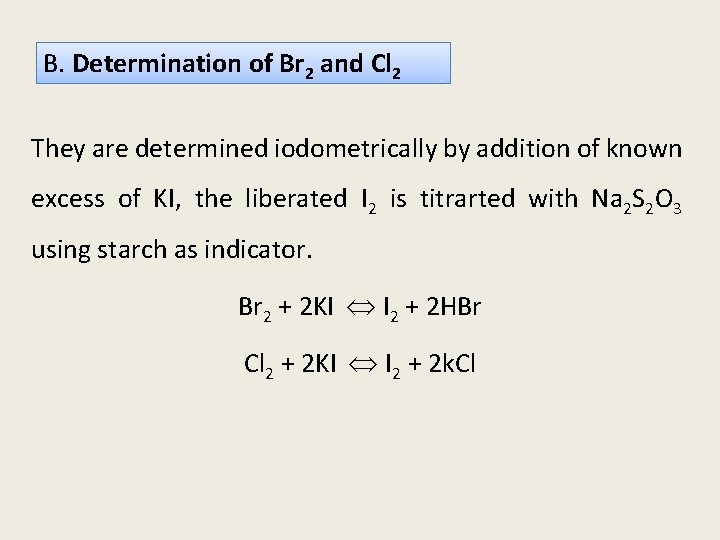
B. Determination of Br 2 and Cl 2 They are determined iodometrically by addition of known excess of KI, the liberated I 2 is titrarted with Na 2 S 2 O 3 using starch as indicator. Br 2 + 2 KI I 2 + 2 HBr Cl 2 + 2 KI I 2 + 2 k. Cl

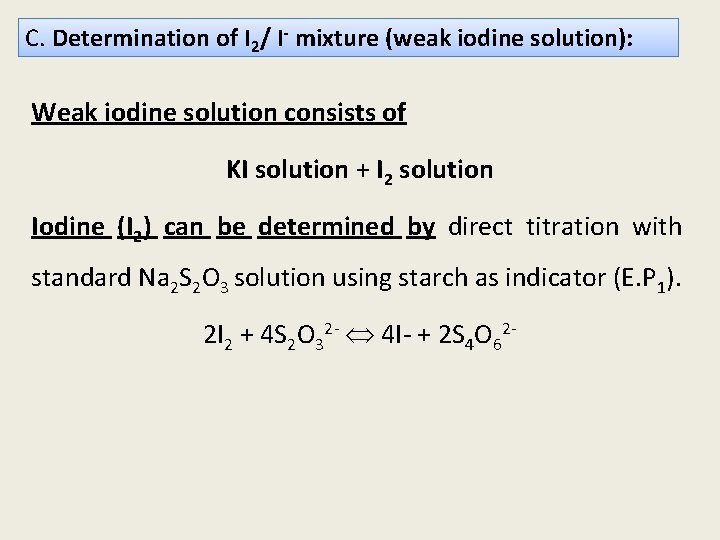
C. Determination of I 2/ I- mixture (weak iodine solution): Weak iodine solution consists of KI solution + I 2 solution Iodine (I 2) can be determined by direct titration with standard Na 2 S 2 O 3 solution using starch as indicator (E. P 1). 2 I 2 + 4 S 2 O 32 - 4 I- + 2 S 4 O 62 -

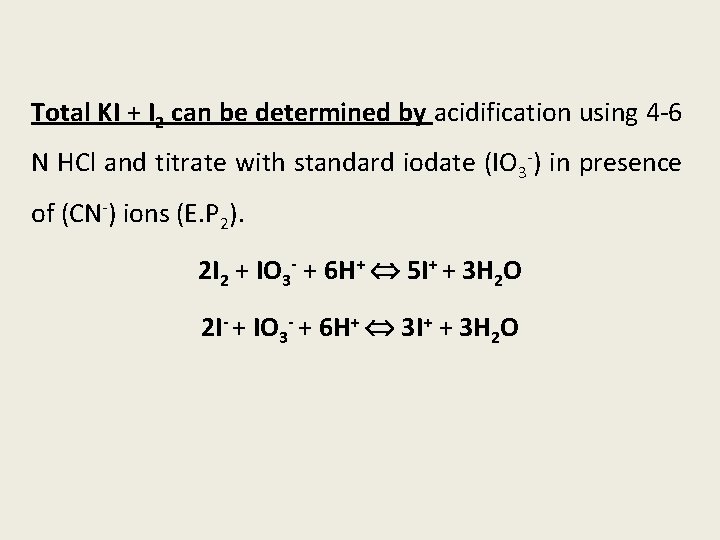
Total KI + I 2 can be determined by acidification using 4 -6 N HCl and titrate with standard iodate (IO 3 -) in presence of (CN-) ions (E. P 2). 2 I 2 + IO 3 - + 6 H+ 5 I+ + 3 H 2 O 2 I- + IO 3 - + 6 H+ 3 I+ + 3 H 2 O
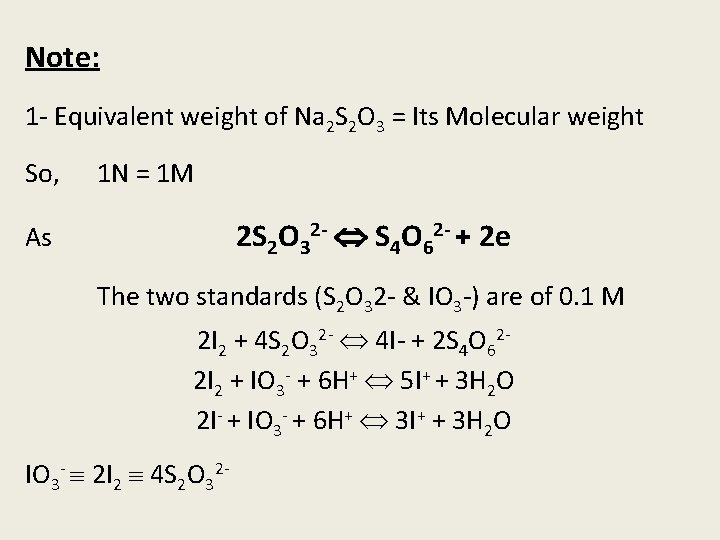
Note: 1 - Equivalent weight of Na 2 S 2 O 3 = Its Molecular weight So, 1 N = 1 M 2 S 2 O 32 - S 4 O 62 - + 2 e As The two standards (S 2 O 32 - & IO 3 -) are of 0. 1 M 2 I 2 + 4 S 2 O 32 - 4 I- + 2 S 4 O 622 I 2 + IO 3 - + 6 H+ 5 I+ + 3 H 2 O 2 I- + IO 3 - + 6 H+ 3 I+ + 3 H 2 O IO 3 - 2 I 2 4 S 2 O 32 -

Reading of Na 2 S 2 O 3 4 reading of IO 3 E. P 1 = X 1 in Na 2 S 2 O 3 scale E. P 2 = X 2 in IO 3 - scale So, we cannot substract E. P 1 - E. P 2 to obtain the KI only. So we must convert the E. P 2 to be in Na 2 S 2 O 3 scale E. P 1 in Na 2 S 2 O 3 scale = 4 X 2 I- = 4 X 2 - X 1 2. Strong iodine solution is determined as the same procedures of weak iodine solution after diluting 25 ml of the solution to 100 ml with ethanol (90 %)

D. Determination of chlorine water Chlorine water system contain some dissolved Cl 2 + hypochlorous acid (HOCl) is formed by the reaction with water. Cl 2 + H 2 O HOCl + HCl Both Cl 2 and HOCl are oxidizing agents for I-. So, they are determined iodometrically by use of known excess of KI where an equivalent amount of I 2 is liberated and can be titrated with standard thiosulphate using starch as indicator. Cl 2 + 2 I I 2 + 2 Cl 2 HOCl + 2 I- + H+ I 2 + 2 Cl- + H 2 O

E. Determination of hypochlorites (Cl. O-): Cl. O- + I- IO- + Cl 2 H+ + IO- + I- I 2 + H 2 O ØBy adding of known excess of KI to the hypochlorite solution acidified with diluted acid ØThe liberated I 2 is titrated with S 2 O 32 - using starch as indicator.

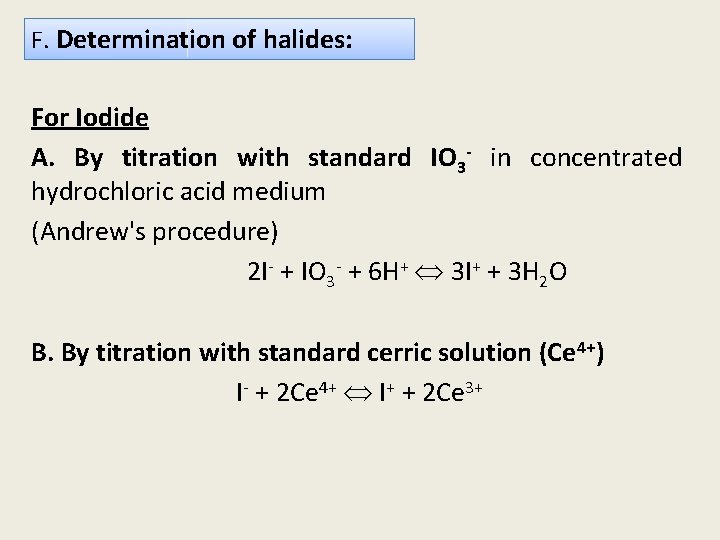
F. Determination of halides: For Iodide A. By titration with standard IO 3 - in concentrated hydrochloric acid medium (Andrew's procedure) 2 I- + IO 3 - + 6 H+ 3 I+ + 3 H 2 O B. By titration with standard cerric solution (Ce 4+) I- + 2 Ce 4+ I+ + 2 Ce 3+

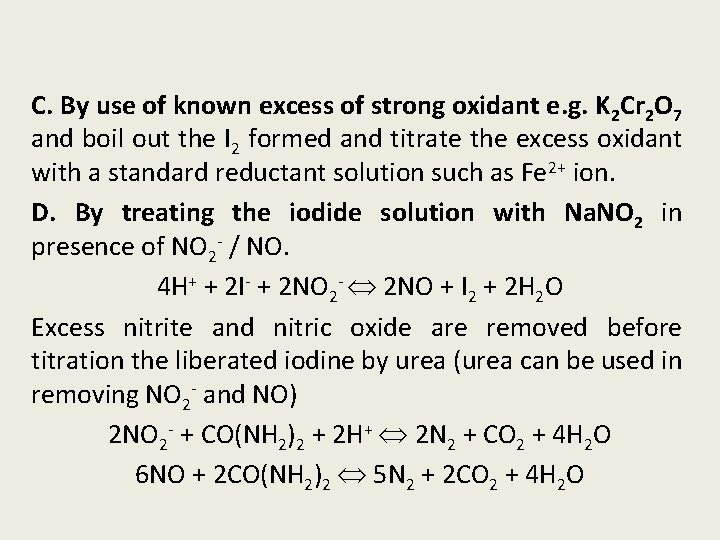
C. By use of known excess of strong oxidant e. g. K 2 Cr 2 O 7 and boil out the I 2 formed and titrate the excess oxidant with a standard reductant solution such as Fe 2+ ion. D. By treating the iodide solution with Na. NO 2 in presence of NO 2 - / NO. 4 H+ + 2 I- + 2 NO 2 - 2 NO + I 2 + 2 H 2 O Excess nitrite and nitric oxide are removed before titration the liberated iodine by urea (urea can be used in removing NO 2 - and NO) 2 NO 2 - + CO(NH 2)2 + 2 H+ 2 N 2 + CO 2 + 4 H 2 O 6 NO + 2 CO(NH 2)2 5 N 2 + 2 CO 2 + 4 H 2 O

For Bromide ØBr- determined by treatment with excess strong oxidant (e. g. K 2 Cr 2 O 7) Br 2 is produced ØDistilled into KI solution, thus the liberating I 2 is titrated with standard Na 2 S 2 O 3 solution
 Sophomoric humor meaning
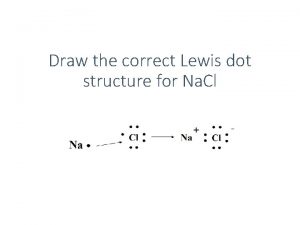
Sophomoric humor meaning Dot structure for na
Dot structure for na Ground state electron configuration of arsenic
Ground state electron configuration of arsenic Lewis structure for arsenic
Lewis structure for arsenic Ionic binary
Ionic binary Ground state of arsenic
Ground state of arsenic Self sealability test
Self sealability test 1806 valentin ross
1806 valentin ross Formula for arsenic pentabromide
Formula for arsenic pentabromide Atom size periodic table
Atom size periodic table Shorthand electron configuration for arsenic
Shorthand electron configuration for arsenic Bismuth nitrite
Bismuth nitrite Sono diagnostic center kushtia
Sono diagnostic center kushtia 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Y chromosome traits
Y chromosome traits Heterogametic
Heterogametic Control determination
Control determination Price and output determination under oligopoly
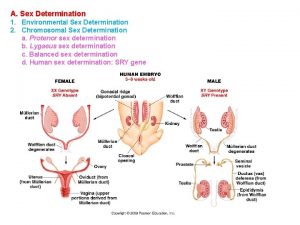
Price and output determination under oligopoly Sex determination and sex linkage
Sex determination and sex linkage