KING FAHD UNIVERSITY OF PETROLEUM AND MINERALS PYP






- Slides: 17

KING FAHD UNIVERSITY OF PETROLEUM AND MINERALS PYP 001 Fall 2012 (Term 121) Chapter 5 The Atom

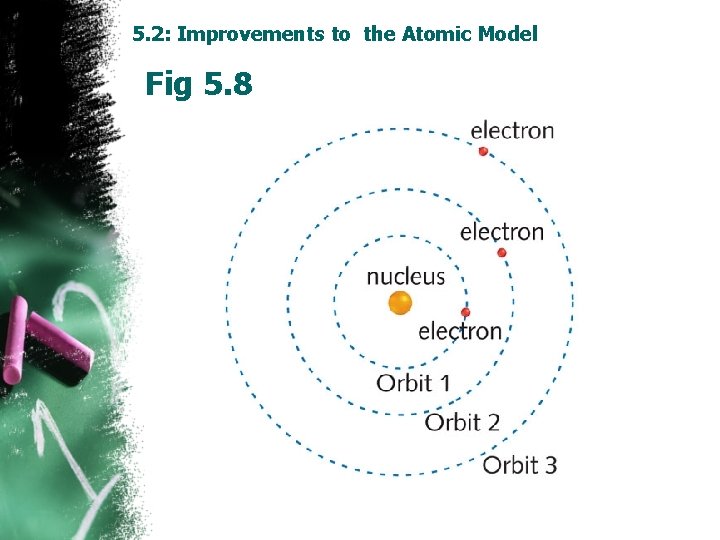
5. 2: Improvements to the Atomic Model Rutherford Theory: failed in describing the position of electrons in an atom. Bohr’s Atomic Model, Ø Electrons revolve around the nucleus at orbits with fixed orbits energy level with fixed distance from the nucleolus. Ø Electrons could not exist between energy levels. Ø Electrons don’t loose energy while travelling in the same energy level. Planck’s theory: Energy exists in tiny packets. • What happen when Electron absorbs a certain amount of energy?

5. 2: Improvements to the Atomic Model Fig 5. 8

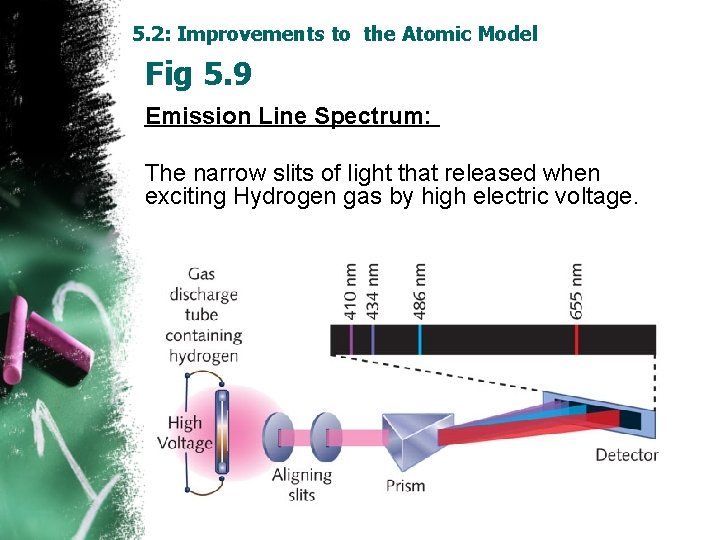
5. 2: Improvements to the Atomic Model Fig 5. 9 Emission Line Spectrum: The narrow slits of light that released when exciting Hydrogen gas by high electric voltage.

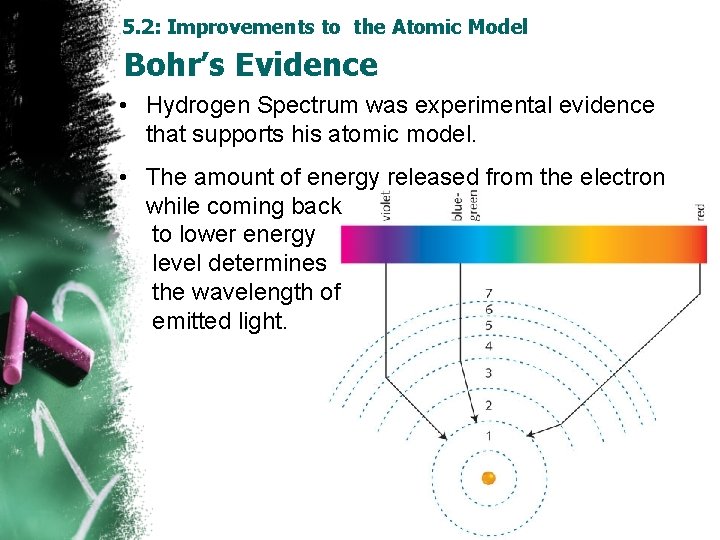
5. 2: Improvements to the Atomic Model Bohr’s Evidence • Hydrogen Spectrum was experimental evidence that supports his atomic model. • The amount of energy released from the electron while coming back to lower energy level determines the wavelength of emitted light.

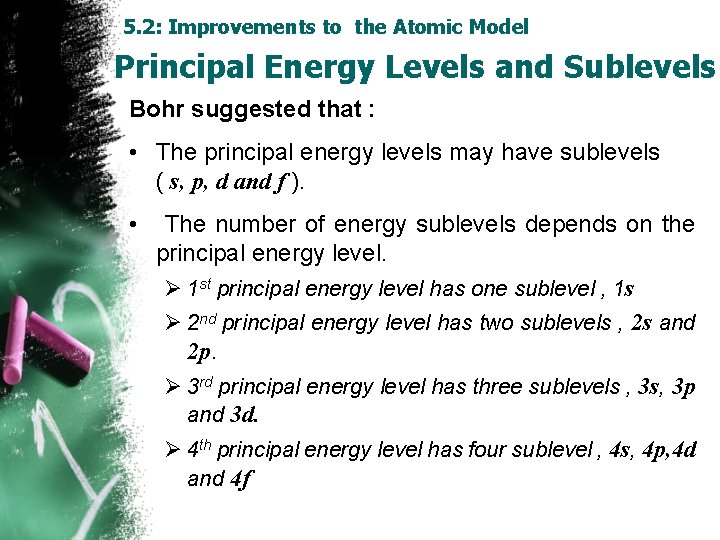
5. 2: Improvements to the Atomic Model Principal Energy Levels and Sublevels Bohr suggested that : • The principal energy levels may have sublevels ( s, p, d and f ). • The number of energy sublevels depends on the principal energy level. Ø 1 st principal energy level has one sublevel , 1 s Ø 2 nd principal energy level has two sublevels , 2 s and 2 p. Ø 3 rd principal energy level has three sublevels , 3 s, 3 p and 3 d. Ø 4 th principal energy level has four sublevel , 4 s, 4 p, 4 d and 4 f

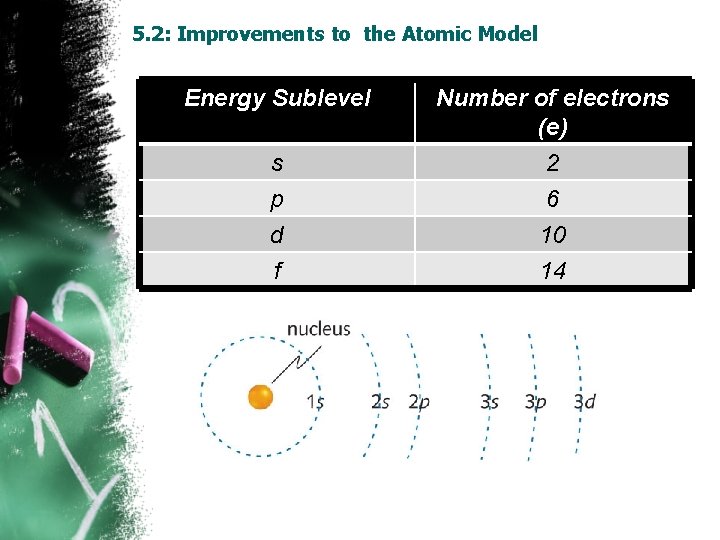
5. 2: Improvements to the Atomic Model Energy Sublevel s p Number of electrons (e) 2 6 d f 10 14

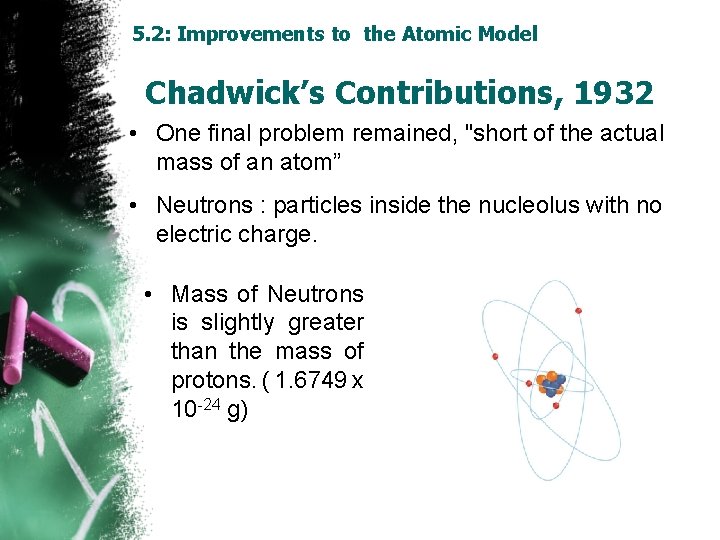
5. 2: Improvements to the Atomic Model Chadwick’s Contributions, 1932 • One final problem remained, "short of the actual mass of an atom” • Neutrons : particles inside the nucleolus with no electric charge. • Mass of Neutrons is slightly greater than the mass of protons. ( 1. 6749 x 10 -24 g)

5. 2: Improvements to the Atomic Model Modern Atomic Theory Heisenberg, 1927 • Its impossible to know both the exact position and exact velocity of an electron in the same time. • Electron Cloud: the region around the nucleus in which the electrons are likely to exist at any specific time.

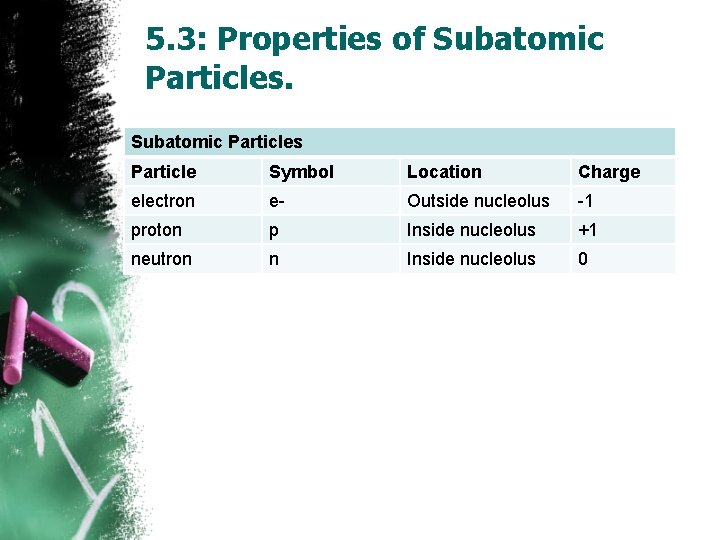
5. 3: Properties of Subatomic Particles Particle Symbol Location Charge electron e- Outside nucleolus -1 proton p Inside nucleolus +1 neutron n Inside nucleolus 0

5. 3: Properties of Subatomic Particles. Atomic Size: • Described in a very small unit “nanometer” , 10 -9. • AVG Atom diameter is about 0. 2 nm. • We can get an image of an atoms through a device called : scanning tunneling microscope.

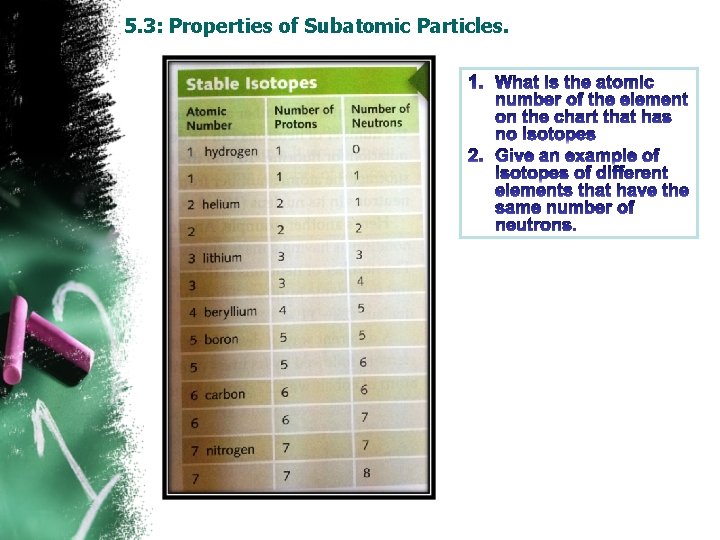
5. 3: Properties of Subatomic Particles. Atomic Number: • The number of protons in the nucleolus is known as “atomic number”. • Every element has a unique atomic number. • Example: atomic number for Au is 79, He is 2. Isotopes : • Atoms of the same element that have a different number of neutrons in their nuclei are called “isotopes”. • “H” has two stable isotopes protium ( 1 p, 0 n) and deuterium (1 p, 1 n), one unstable isotope tritium (1 p, 2 n).

5. 3: Properties of Subatomic Particles.

5. 3: Properties of Subatomic Particles. Mass Number • The total number of protons and neutrons in the nucleus of an atom is called “mass number”. • Atomic notation : Mass number Ø Fluorine Atomic number • Number of p = • Number of e = • Number of N= • Also you can write it as ( Fluorine-19).

5. 3: Properties of Subatomic Particles. Atomic Mass: • (amu) : atomic mass unit is the unit that used to describe atomic masses. • 1 amu= 1/12 of the mass of carbon-12 atom. • The atomic mass of an element is the weighted average of all naturally occurring isotopes. • Example: calculating atomic mass of Carbon: Carbon Ø C-12 : 12. 00000 amu x ( 98. 89%) = 11. 87 amu Ø C-13 : 13. 00335 amu x ( 1. 11%) = 0. 144 amu. Ø Total = 11. 87+0. 144 = 12. 01 amu

Summary: • 5. 2: Improvements to the Atomic Model • Bohr proposed a model of the atom that suggested that electrons orbit the nucleolus in discreet energy levels. • Chadwick discovered the neutron, which is a subatomic particle that is eclectically neutral. • Acceding to the modern theory of the atom the specific locations of electrons can’t determined. Instead a region in which they are likely to exist, called an electron cloud, can be described. • .

Summary: • 5. 3: Properties of Subatomic Particles. • Protons, neutrons, and electrons are described as subatomic particles. Protons and neutrons make up the nucleus of an atom. Electrons are located outside the nucleus. • The atomic number of an atom is the number of protons in the nucleus. • The sum of the numbers of protons and neutrons in an atom’s nucleus is the atom’s mass number. • Two atoms of the same element with different numbers of neutrons are known as isotopes. Each isotope exists naturally in a different abundance, so the atomic mass of an element is calculated as weighted average.
 Milk alkali syndrome
Milk alkali syndrome Fahd: hi, ryan. where are you going?
Fahd: hi, ryan. where are you going? Fuente del rey fahd
Fuente del rey fahd Ufa state petroleum technical university
Ufa state petroleum technical university Ict scope and sequence pyp
Ict scope and sequence pyp Ict scope and sequence pyp
Ict scope and sequence pyp Pyp exhibition presentation ideas
Pyp exhibition presentation ideas Pyp exhibition rubric
Pyp exhibition rubric Prevencin
Prevencin Pyp-001
Pyp-001 Making the pyp happen
Making the pyp happen Pyp 004 kfupm
Pyp 004 kfupm Pyp-001
Pyp-001 Site structure
Site structure Pyp transdisciplinary themes who we are
Pyp transdisciplinary themes who we are Pyp exhibition timeline
Pyp exhibition timeline Ib pyp
Ib pyp Eca rto manager
Eca rto manager