KING FAHD UNIVERSITY OF PETROLEUM AND MINERALS PYP







- Slides: 12

KING FAHD UNIVERSITY OF PETROLEUM AND MINERALS PYP 001 Fall 2012 (Term 121) Chapter 2 Types of Matter

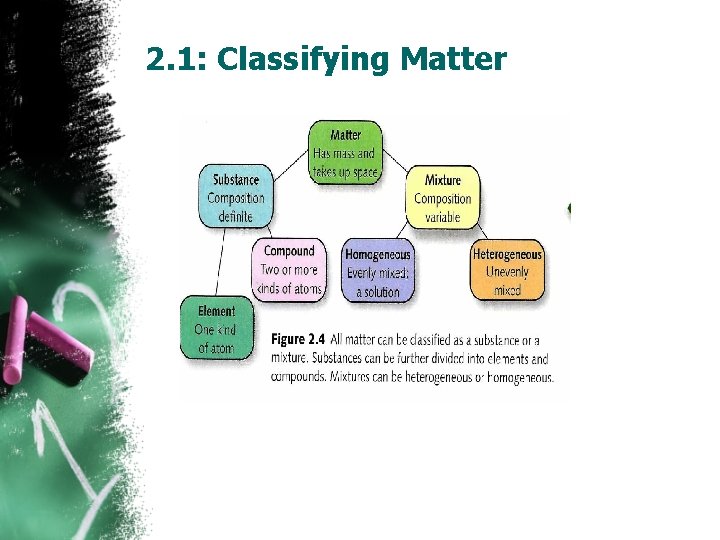
2. 1: Classifying Matter • Matter: is any thing that has mass and takes up space. (Ex. Dish, paper, money…. . Air ”not visible” ) • Not matter(Ex. Light, heat and sounds). • Pure substance • All matter is made up of small particles called atoms. • Molecules: is formed when two or more atoms combine. • Pure substance: is a type of matter made up of only one kind of particle. (can be element or compound)

2. 1: Classifying Matter • Elements: A substance in which all of the atoms are the same is called a chemical element. (Gold, silver, Iron, …. ) • Compounds: is a pure substance in which the atoms two or more elements combine. (Carbon dioxide, Ammonia, table salt, rust, water…. ) the elements in a compound always combine in a specific ratio. • Mixtures: (fruit salad) is made up of substance that can be separated by physical means. Unlike compounds, mixtures do not always contain the same ratio of the substances that make them up.

2. 1: Classifying Matter • Heterogeneous mixture: A mixture in which the different materials can be distinguished easily(Granite). • Homogeneous mixture: “Solution”: a mixture in which the substances are uniformly spread out. (air, oacen water, antifreeze and tea)

2. 1: Classifying Matter

2. 2: Elements Discovery of Elements • Today Scientists identify 117 different elements. Of those, 94 are found in nature, 23 elements can be made in laboratories. They are unstable and exist for only short periods of time. • The ancient Greeks described only four elementsearth, fire, water and air. They believed that various combinations of these four elements could form everything on the earth. ”lava-earth and fire” Describing Elements: Elements are represented by chemical symbol consist of one or two letters”abbreviation”. The first letter is always capitalized. (C, He…)(Iron ”ferrum”: Fe)

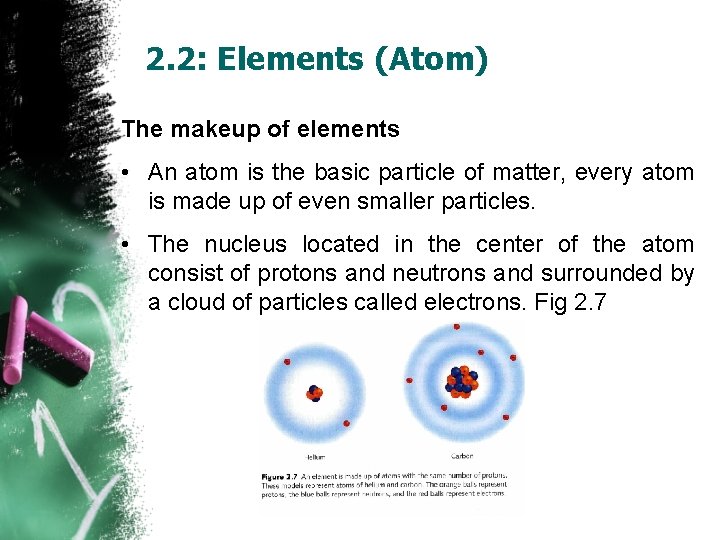
2. 2: Elements (Atom) The makeup of elements • An atom is the basic particle of matter, every atom is made up of even smaller particles. • The nucleus located in the center of the atom consist of protons and neutrons and surrounded by a cloud of particles called electrons. Fig 2. 7

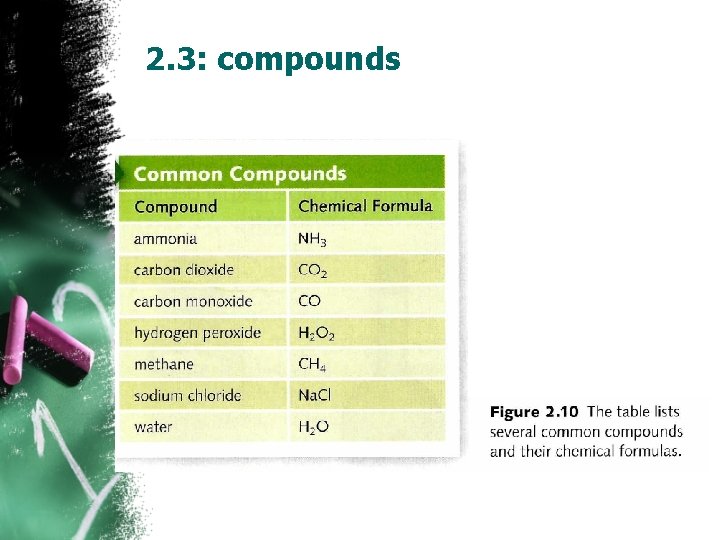
2. 3: compounds Properties of compounds • The properties of compounds are different from the properties of the elements from which they form. (Na. Cl – save as salt, chlorine is poisonous gas) • (water is an odorless compound H 2 O-hydrogen peroxide H 2 O 2 it has strong odor) composition of compounds(makeup) “H 2 O” single drop of water, a full bottle, or a pool full of water all water has the same composition Chemical formulas: compounds can be represented by chemical formulas which shows the elements in a compound and the ratio of their atoms. Fig 2. 10 • A subscript below an element’s symbol indicates the number of atom of that element present in the compound.

2. 3: compounds

2. 3: compounds Separating compounds • Compounds cannot be decomposed, or separated into individual elements, by physical means such as evaporation or filtering. Instead, a more complex chemical process is required. One such process is used to change water (H 2 O) into hydrogen and oxygen. The process is known as electrolysis. • Other compounds can be broken down in a similar way. However, for some the process requires several steps. When chalk, Ca. Co 3, is heated, it decomposes into lime, Ca. O, and carbon dioxide, Co 2. Neither of these products are elements. They are still compounds. Through electrolysis, lime can be broken down into calcium, Ca, and oxygen, O. carbon dioxide can then be broken down into carbon, C and oxygen, O.

Summary • Classifying matter • Matter is anything that has mass and takes up space • Matter can be classified as a pure substance or a mixture • Pure substances include elements and compounds. Mixtures can be heterogeneous • Elements • Elements are defined as pure substances made up of only one type atom. • Elements are represented with chemical symbols

Summary • Compounds • A compound is a pure substance made up of a combination of two or more different elements that exist in specific ratios. • Compounds are made of combination of atoms called molecules • Chemical formulas made up of letters and subscripts are used to represent the ratio of atoms of combining elements in compounds. • Compounds can be broken down into elements through chemical process such as electrolysis. • Mixtures • A mixture is a blend of substances that keep their individual identities and can exist in varying ratios. • The substances in a heterogeneous mixture can be easily identified. The substances in a homogenous mixture become so uniformly mixed that they cannot be recognized individually. • Mixtures can be separated using processers such as filtration, distillation, paper chromatography, and evaporation.