Kinetic Theory and Density Ch 3 pg 74





- Slides: 10

Kinetic Theory and Density Ch. 3 pg. 74 -75

Balloon Demo • Pass around the balloon and sniff it. What do you think is in the balloon? (Don’t shout it out!) • Are the particles inside the balloon matter? How do you know? • How were you able to smell the particles inside the balloon?

Kinetic Theory • The kinetic theory describes how matter behaves and the differences between solids, liquids, and gases. • There are 3 main points to theory: – 1. All matter is made up of atoms and molecules that act as tiny particles. – 2. All particles are in motion. The higher the temperature, the faster they move. – 3. At the same temperature, heavier (more massive) particles will move more slowly.

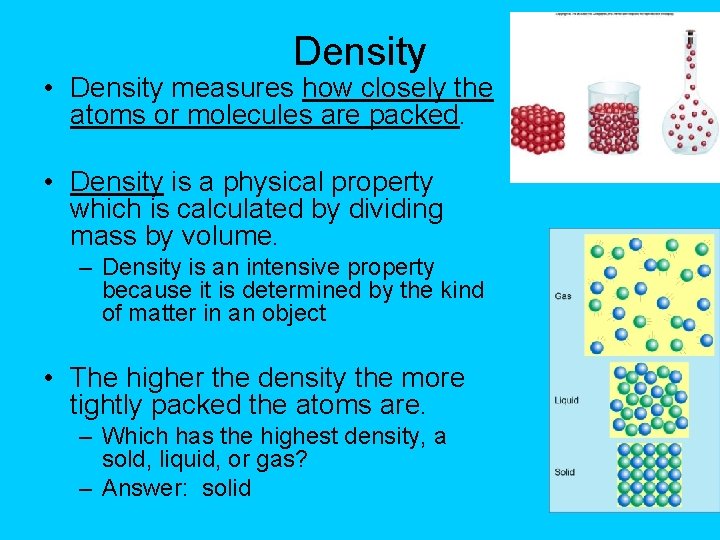
Density • Density measures how closely the atoms or molecules are packed. • Density is a physical property which is calculated by dividing mass by volume. – Density is an intensive property because it is determined by the kind of matter in an object • The higher the density the more tightly packed the atoms are. – Which has the highest density, a sold, liquid, or gas? – Answer: solid

Density (continued) • Density can help determine if a substance will float or sink in another substance. – The volume of most substances increases as the temperature increases, however the mass stays the same. The density of a substance will decrease as the temperature increases • The units of density are grams per cubic centimeter or grams per milliliter. units: _g_ or _g_ cm 3 m. L

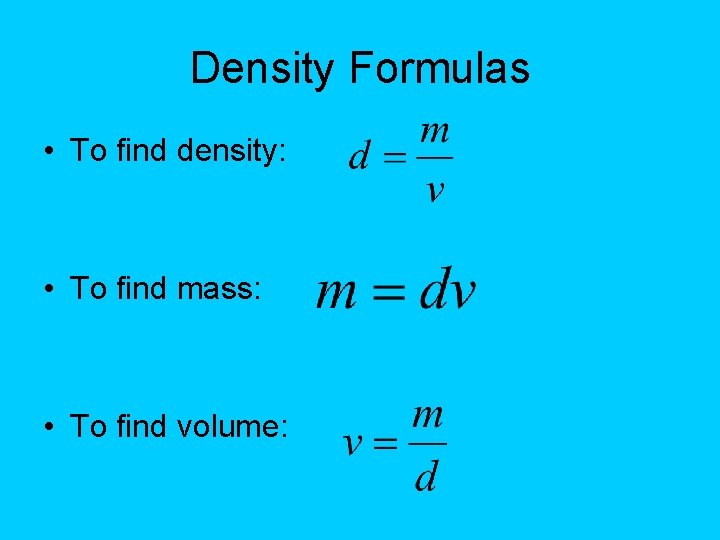
Density Formulas • To find density: • To find mass: • To find volume:

Buoyancy • Buoyancy occurs when a more dense substance pushes a less dense substance upwards to floating.

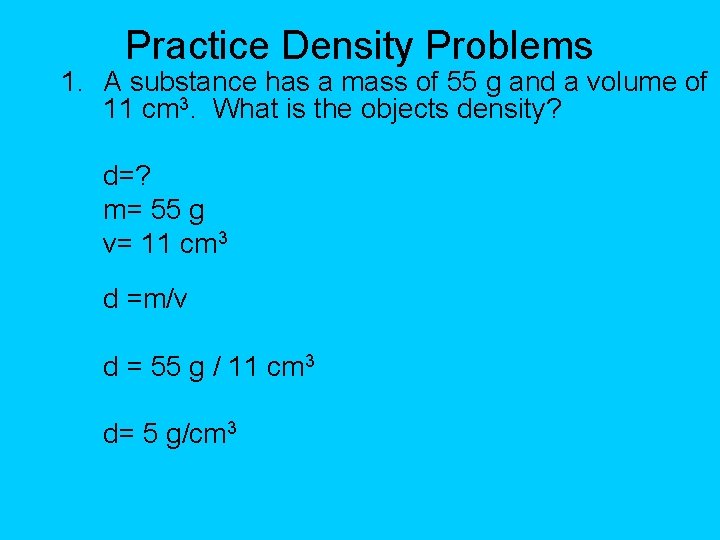
Practice Density Problems 1. A substance has a mass of 55 g and a volume of 11 cm 3. What is the objects density? d=? m= 55 g v= 11 cm 3 d =m/v d = 55 g / 11 cm 3 d= 5 g/cm 3

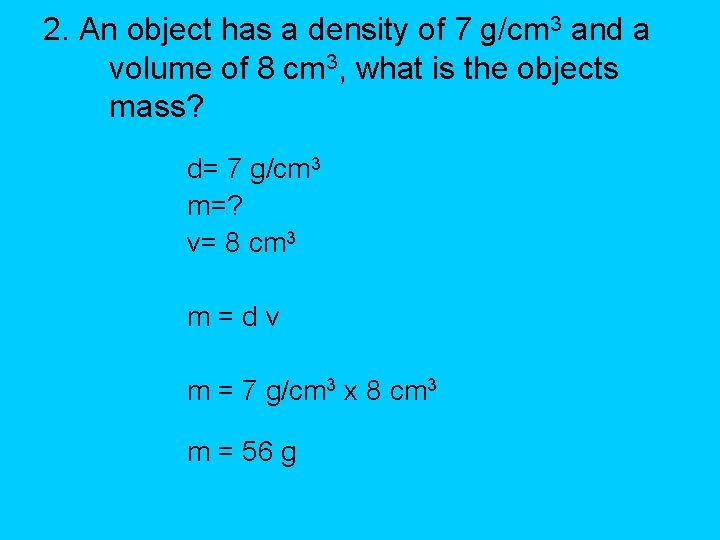
2. An object has a density of 7 g/cm 3 and a volume of 8 cm 3, what is the objects mass? d= 7 g/cm 3 m=? v= 8 cm 3 m=dv m = 7 g/cm 3 x 8 cm 3 m = 56 g

3. An object has a density of 5 g/cm 3 and a mass of 25 g, what is the objects volume? d= 5 g/cm 3 m= 25 g v= ? v= m/d v= 25 g / 5 g/cm 3 v= 5 cm 3