ISOTS 19218 WHO Informal Consultation on Nomenclatures for






- Slides: 17

ISO/TS 19218 WHO Informal Consultation on Nomenclatures for Medical Devices March 23 -24, 2011 Leighton Hansel Convener ISO/TC 210 WG 3 Symbols and Nomenclature for Medical Devices Director, Regulatory Affairs Abbott Quality and Regulatory Abbott Laboratories

1999 Proposed NWIP • Standard for coding device failures suggested to ISO/TC 210 WG 3, Symbols and Nomenclature for Medical Devices by FDA/GHTF SG 2 • To support adverse event information exchange between regulatory authorities • Manufacturer submissions of adverse event reports to regulatory authorities. • Could also be used by end users • Excluded patient outcomes • For post market events, not for clinical studies 2

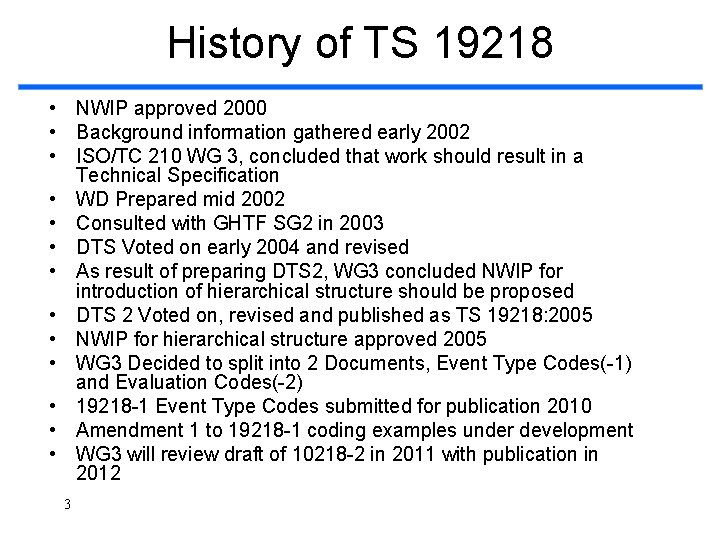
History of TS 19218 • NWIP approved 2000 • Background information gathered early 2002 • ISO/TC 210 WG 3, concluded that work should result in a Technical Specification • WD Prepared mid 2002 • Consulted with GHTF SG 2 in 2003 • DTS Voted on early 2004 and revised • As result of preparing DTS 2, WG 3 concluded NWIP for introduction of hierarchical structure should be proposed • DTS 2 Voted on, revised and published as TS 19218: 2005 • NWIP for hierarchical structure approved 2005 • WG 3 Decided to split into 2 Documents, Event Type Codes(-1) and Evaluation Codes(-2) • 19218 -1 Event Type Codes submitted for publication 2010 • Amendment 1 to 19218 -1 coding examples under development • WG 3 will review draft of 10218 -2 in 2011 with publication in 2012 3

Initial Version of TS 19218 • Development based on two documents – FDA Coding Evaluation Report – An Evaluation of the Coding System for Device Problems and Patient Effects Used to Report Adverse Medical Device Events to the FDA Med. Watch Program – Report on Medical Device Fault Conditions (NKKN, Haukeland University Hospital) 4

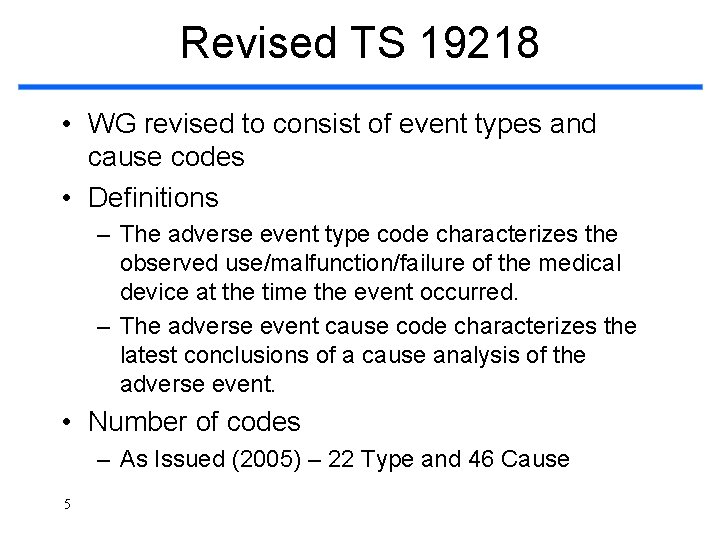
Revised TS 19218 • WG revised to consist of event types and cause codes • Definitions – The adverse event type code characterizes the observed use/malfunction/failure of the medical device at the time the event occurred. – The adverse event cause code characterizes the latest conclusions of a cause analysis of the adverse event. • Number of codes – As Issued (2005) – 22 Type and 46 Cause 5

Device vs. Drug Report Numbers 6

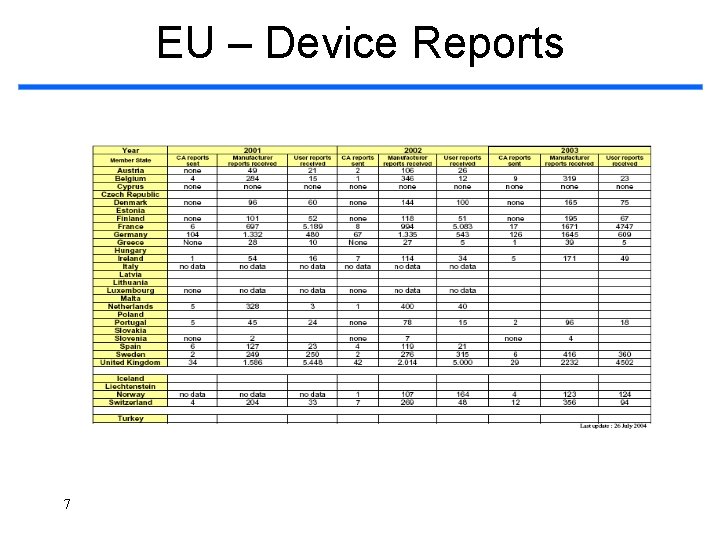
EU – Device Reports 7

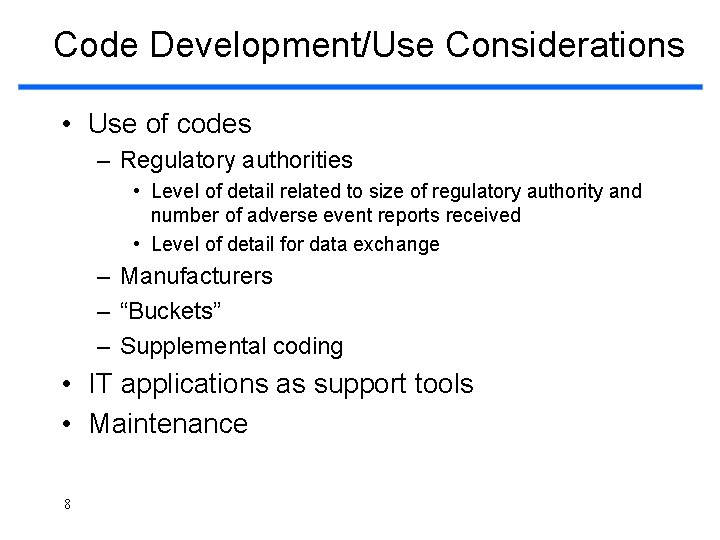
Code Development/Use Considerations • Use of codes – Regulatory authorities • Level of detail related to size of regulatory authority and number of adverse event reports received • Level of detail for data exchange – Manufacturers – “Buckets” – Supplemental coding • IT applications as support tools • Maintenance 8

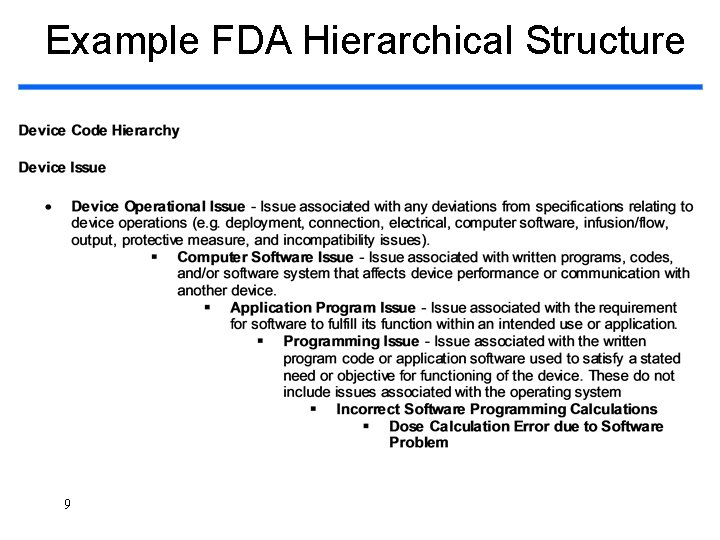
Example FDA Hierarchical Structure 9

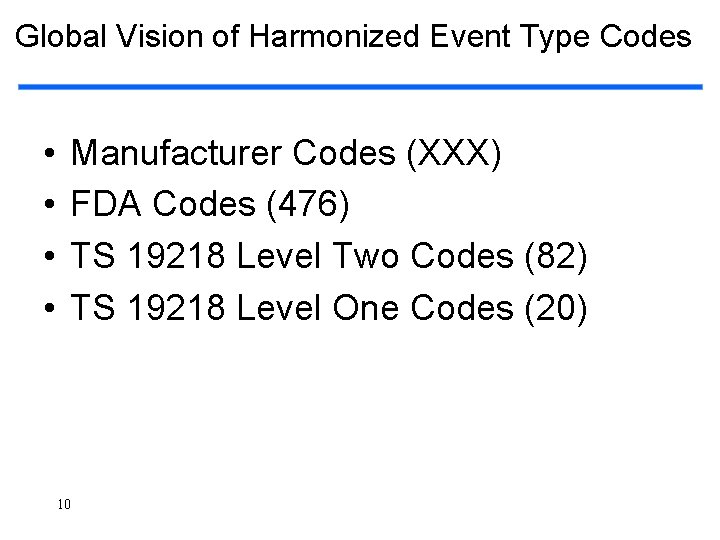
Global Vision of Harmonized Event Type Codes • • Manufacturer Codes (XXX) FDA Codes (476) TS 19218 Level Two Codes (82) TS 19218 Level One Codes (20) 10

19218 -1 Event Type Codes Level 1 11

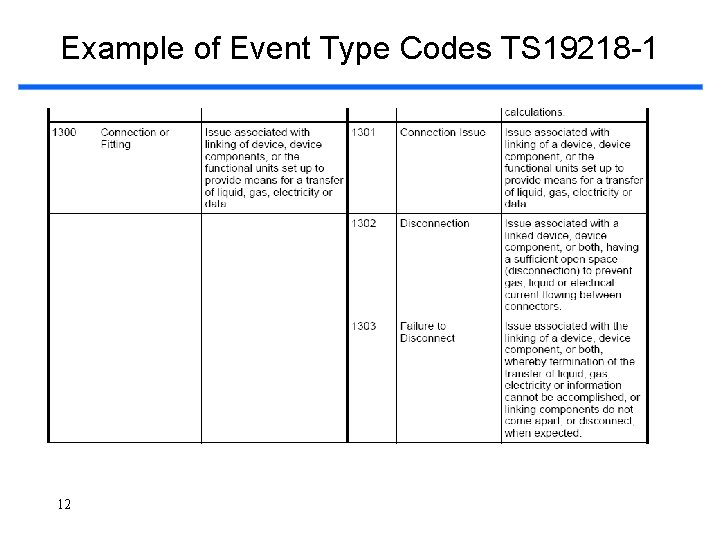
Example of Event Type Codes TS 19218 -1 12

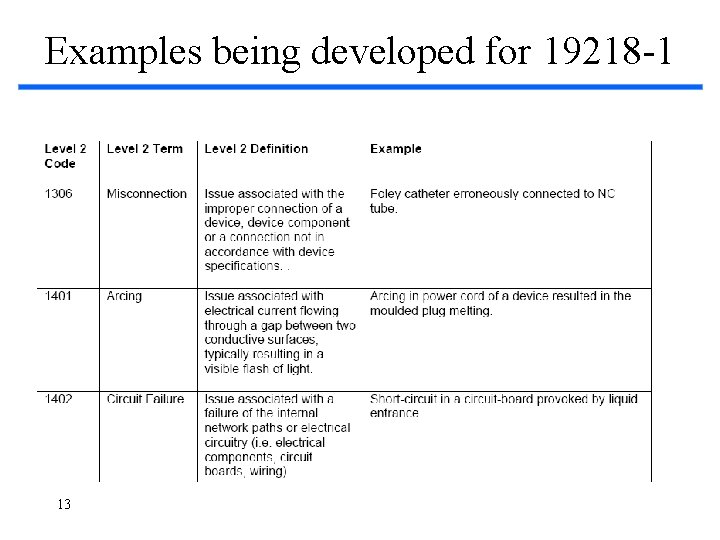
Examples being developed for 19218 -1 13

19218 -2 vs. 19218: 2005 14

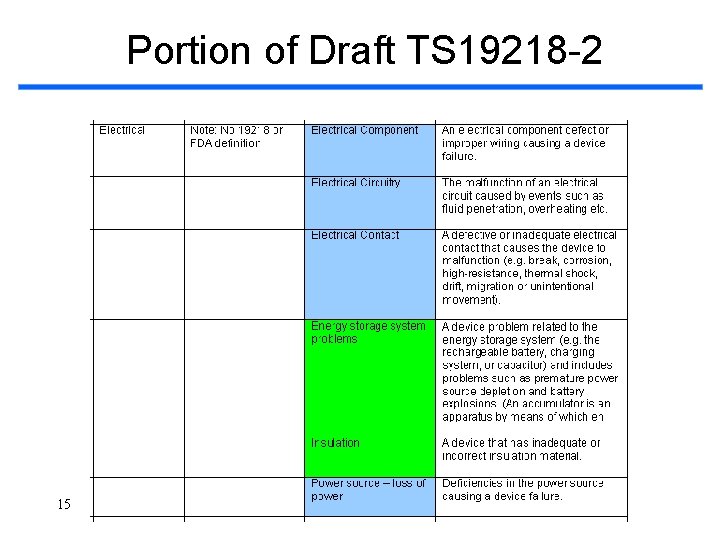
Portion of Draft TS 19218 -2 15

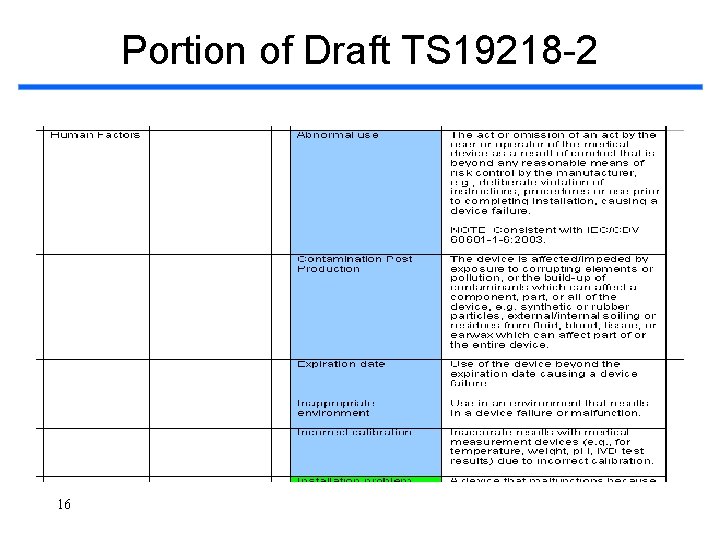
Portion of Draft TS 19218 -2 16

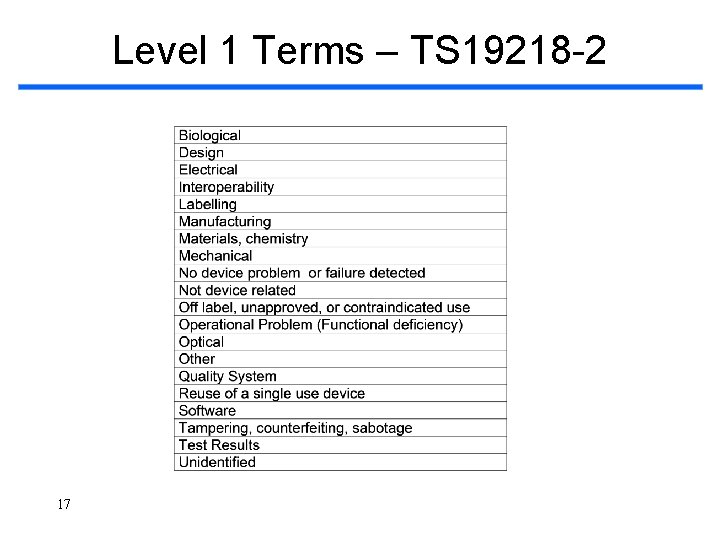
Level 1 Terms – TS 19218 -2 17
 Iso ts 19218
Iso ts 19218 Sconet nomenclatures
Sconet nomenclatures Gestion des nomenclatures
Gestion des nomenclatures Sconet
Sconet Inculta formal
Inculta formal Ekologiskt fotavtryck
Ekologiskt fotavtryck Cks
Cks Läkarutlåtande för livränta
Läkarutlåtande för livränta Klassificeringsstruktur för kommunala verksamheter
Klassificeringsstruktur för kommunala verksamheter Tack för att ni lyssnade
Tack för att ni lyssnade Inköpsprocessen steg för steg
Inköpsprocessen steg för steg Påbyggnader för flakfordon
Påbyggnader för flakfordon Egg för emanuel
Egg för emanuel Tack för att ni har lyssnat
Tack för att ni har lyssnat Tidbok för yrkesförare
Tidbok för yrkesförare Rutin för avvikelsehantering
Rutin för avvikelsehantering Var finns arvsanlagen
Var finns arvsanlagen Myndigheten för delaktighet
Myndigheten för delaktighet