Ions Atoms can lose or gain electrons If
- Slides: 9

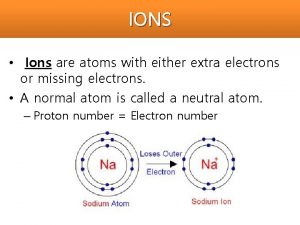
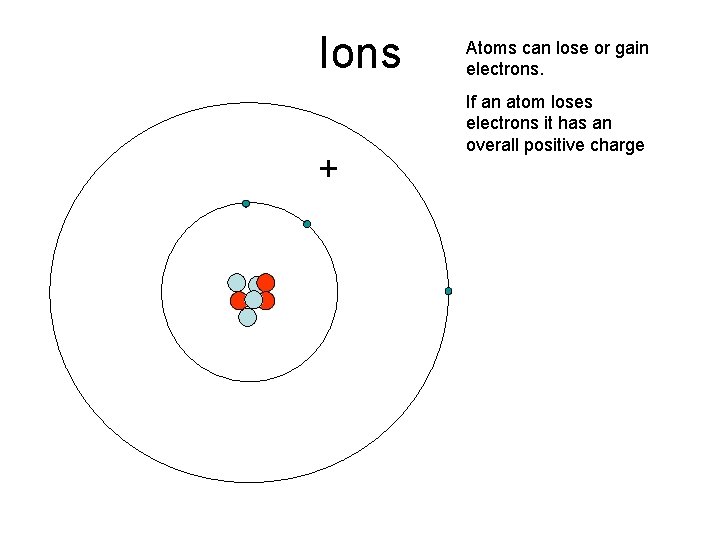
Ions + Atoms can lose or gain electrons. If an atom loses electrons it has an overall positive charge

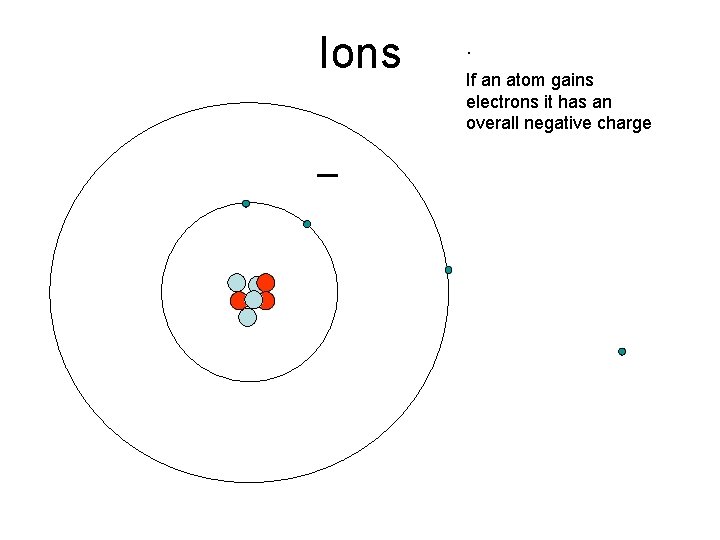
Ions _ . If an atom gains electrons it has an overall negative charge

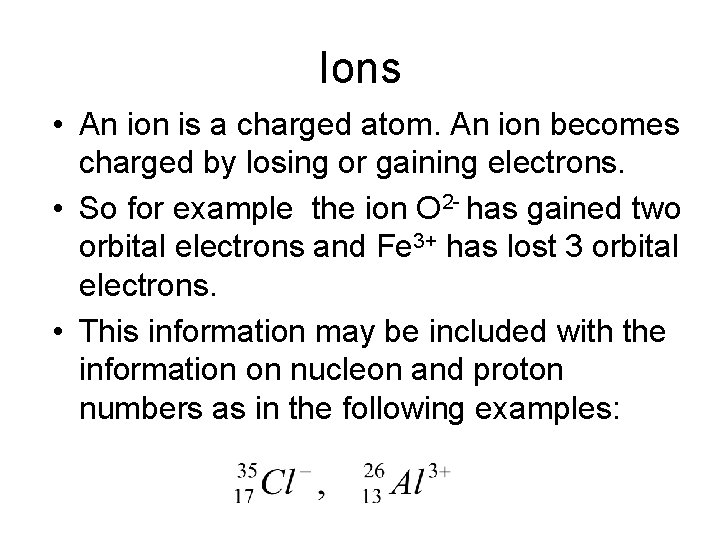
Ions • An ion is a charged atom. An ion becomes charged by losing or gaining electrons. • So for example the ion O 2 - has gained two orbital electrons and Fe 3+ has lost 3 orbital electrons. • This information may be included with the information on nucleon and proton numbers as in the following examples:

Many times we are just concerned with what the nucleus of an atom is like

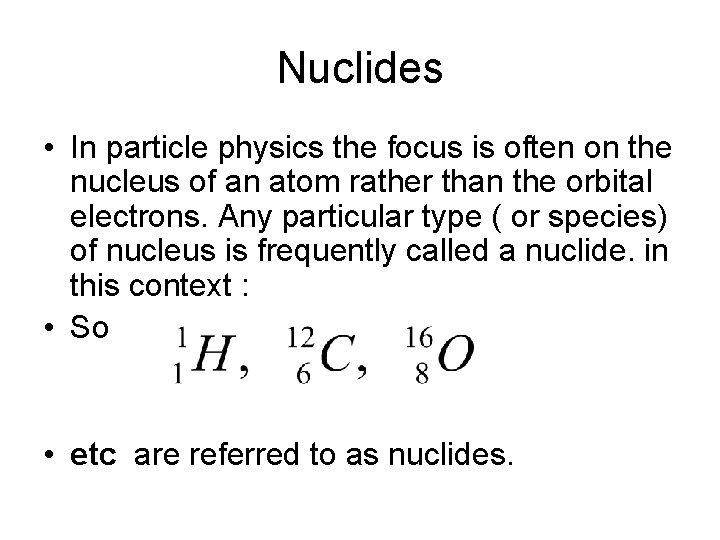
Nuclides • In particle physics the focus is often on the nucleus of an atom rather than the orbital electrons. Any particular type ( or species) of nucleus is frequently called a nuclide. in this context : • So • etc are referred to as nuclides.

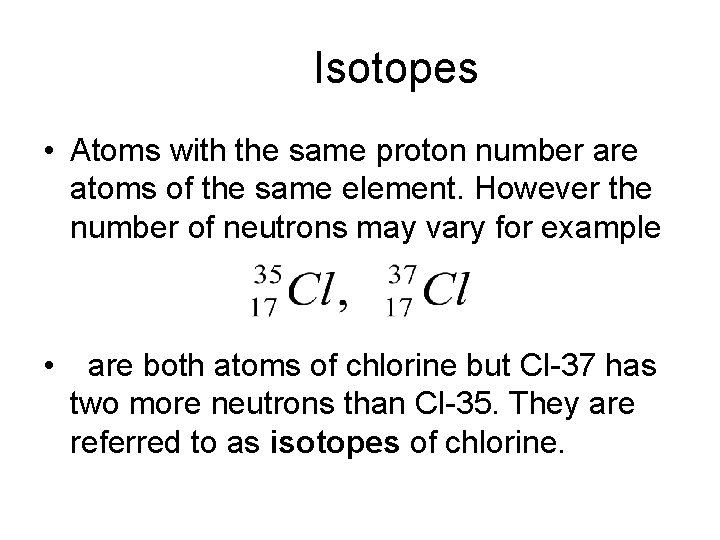
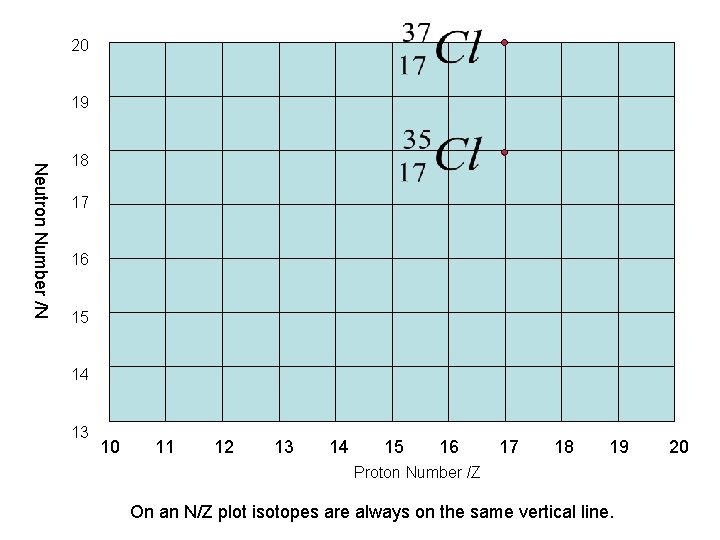
Isotopes • Atoms with the same proton number are atoms of the same element. However the number of neutrons may vary for example • are both atoms of chlorine but Cl-37 has two more neutrons than Cl-35. They are referred to as isotopes of chlorine.

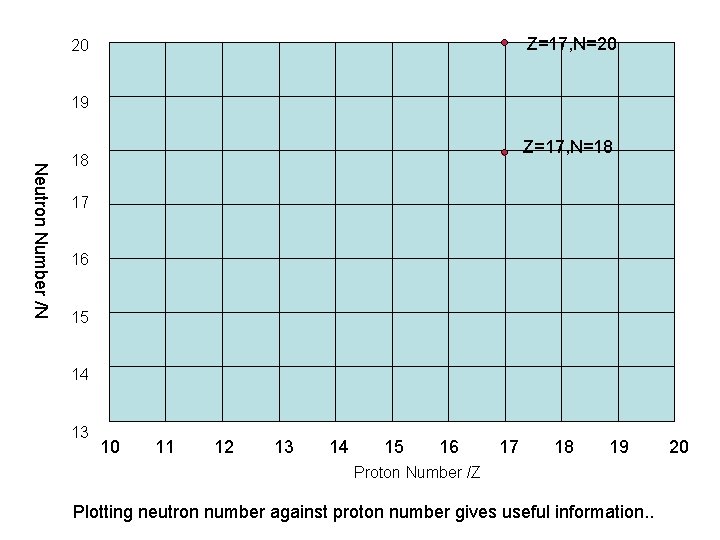
Z=17, N=20 20 19 Z=17, N=18 Neutron Number /N 18 17 16 15 14 13 10 11 12 13 14 15 16 17 18 19 Proton Number /Z Plotting neutron number against proton number gives useful information. . 20

20 19 Neutron Number /N 18 17 16 15 14 13 10 11 12 13 14 15 16 17 18 19 Proton Number /Z On an N/Z plot isotopes are always on the same vertical line. 20

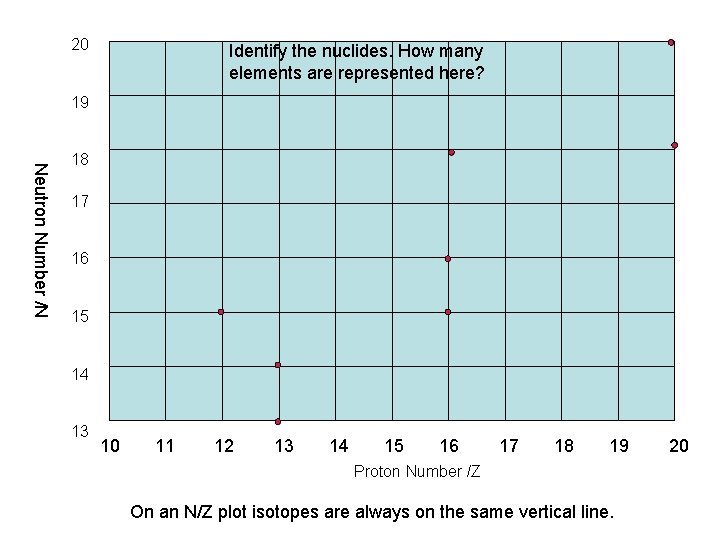
20 Identify the nuclides. How many elements are represented here? 19 Neutron Number /N 18 17 16 15 14 13 10 11 12 13 14 15 16 17 18 19 Proton Number /Z On an N/Z plot isotopes are always on the same vertical line. 20